Context
The incidence and awareness of postprostatectomy incontinence (PPI) has increased during the past few years, probably because of an increase in prostate cancer surgery. Many theories have been postulated to explain the pathophysiology of PPI.
Objective
The current review scrutinizes various pathophysiologic mechanisms underlying the occurrence of PPI.
Evidence acquisition
A search was conducted on PubMed and EMBASE for publications on PPI. The primary search returned 2518 publications. Animal and basic research studies, letters, publications on prostatectomy for benign reasons, pathology of prostatic carcinoma, radiotherapy and hormone therapy of prostatic carcinoma, and review articles were all used as criteria for exclusion from the study. A total of 128 publications were selected for final analysis.
Evidence synthesis
Neuromuscular anatomic elements and pelvic support are known to influence PPI as evidenced by multiple publications. A number of non-anatomic and surgical elements have been postulated as contributing factors to PPI. Biological factors and preoperative parameters include: functional bladder changes, age, body mass index (BMI), pre-existing lower urinary tract symptoms (LUTS), prostate size, and oncologic factors. Multiple studies reported the impact of specific anatomic/surgical factors, including fibrosis, shorter membranous urethral length (MUL), anastomotic stricture, damage to the neurovascular bundle, and extensive dissection, all of which have a negative impact on the continence status of patients following radical prostatectomy (RP). Investigation of the impact of techniques to spare the bladder neck and additional procedures to reconstruct the posterior or anterior support structures (eg, the Rocco stitch) on continence status is ongoing.
Conclusions
Anatomic support and pelvic innervation appear to be important factors in the etiology of PPI. Biological/preoperative factors including greater age at time of surgery, pre-existing LUTS, high BMI, shorter MUL, and functional bladder changes have a negative impact on continence after RP. Extensive dissection during surgery, damage to the neurovascular bundle, and postoperative fibrosis also have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior fixation of the bladder-urethra anastomosis are associated with better continence rates. There is still debate about whether posterior pelvic reconstruction leads to better postoperative continence rates.
Patient summary
Radical prostatectomy is an oncologic procedure and thus requires removal of the entire prostate gland and seminal vesicles, ideally with negative surgical margins. This sometimes results in urinary incontinence. The factors contributing to urinary incontinence are explained in this article.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
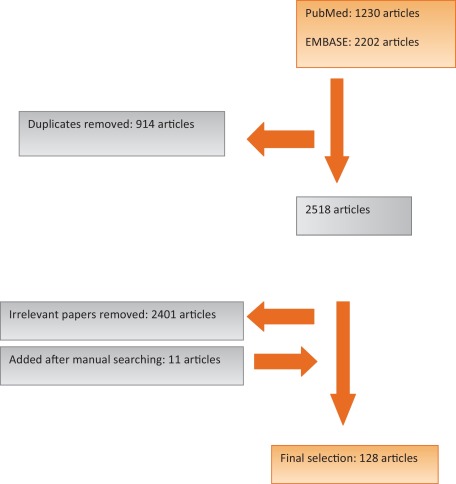
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.
The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.
Persistent urinary incontinence (UI) after radical prostatectomy (RP), commonly referred to as postprostatectomy incontinence (PPI), is an adverse event that leads to significant distress. Rates of PPI vary and depend on the definition of incontinence, severity, bother, and the methodology to assess its magnitude. While multiple factors are associated with the development of PPI, surgical modifications also play a role. The influence of advanced surgical techniques such as laparoscopic robot-assisted RP (RARP) on continence remains a point of debate. With the increase in surgery for prostate cancer, there has been a concomitant increase in the prevalence of PPI and thus greater awareness of the problem. The etiology of PPI is multifactorial and has been the subject of much study.
In a systematic review of more than 8000 men who underwent RARP, laparoscopic prostatectomy, or retropubic prostatectomy, Ficarra et al [1] found that for a “no pad” definition of UI, rates ranged from 4% to 31%, with a mean of 16%. Age, body mass index (BMI), comorbidity index, lower urinary tract symptoms (LUTS), and prostate volume were the most relevant preoperative predictors of UI after RARP. The authors concluded that the prevalence of UI after RARP is influenced by preoperative patient characteristics, surgeon experience, surgical technique, and methods used to collect and report data. The techniques purported to provide better functional results were nerve-sparing procedures, bladder neck preservation, preservation of anterior urethral ligaments, and proper urethrovesical reconstruction. RARP appeared to have better continence rates compared to open prostatectomy, whereas bladder neck preservation resulted in better continence rates compared to bladder neck reconstruction.
The natural history of urinary function recovery after RP is such that most patients regain urinary continence within the first year; however, modest improvement in urinary continence can still be observed through the second year [2].
Knowledge about the anatomy of the urethral sphincter complex and its surrounding structures and innervation in relation to urinary continence is well described in the literature. We provide a brief description of the latest understanding of the anatomy in Section 3. The function of these anatomic structures and their specific role in maintaining urinary continence is much less well understood. The role of these structures in urinary continence is mostly inferred from the effect of applying correcting measures to improve urinary continence. More review manuscripts have been published that elucidate the mechanisms underlying PPI. For example, Loughlin and Prasad [3] concluded that the return of urinary continence after surgery is influenced by multiple factors, including patient selection, technical nuances, and definitions. We performed a review of the literature from the start of RP to document the current state of knowledge with respect to PPI pathophysiology.
Pub Med and EMBASE were searched for publications on PPI from January 1, 1990 to May 20, 2015. We chose 1990 because this year was approximately when RP was first performed. The search terms were: urinary incontinence, urinary stress incontinence, urinary urge incontinence, and RP. Details of search methods are shown in the Supplementary material. The search was limited to publications written in English. The inclusion criteria were source publications (clinical studies) describing risk factors and potential pathologic mechanisms underlying urinary incontinence following RP and their impact on current surgical correction practice. The exclusion criteria were: animal studies; case reports; letters; publications on simple prostatectomy, transurethral resection of the prostate, cryotherapy, laser vaporization, and other less invasive approaches; articles on the pathophysiology of benign prostatic hyperplasia, the pathology of prostatic carcinoma, and radiotherapy and hormonal therapy for prostatic carcinoma; and review articles. The primary search returned 2518 publications, from which 128 articles were selected for final analysis after applying the inclusion and exclusion criteria. A flow diagram of the selection process is shown in Figure 1. The level of evidence (LE) and sample size for studies included in the final analysis are shown in the evidence synthesis to indicate the strength of evidence for each potential PPI contributory factor.

The urethral sphincter complex consists of two functionally independent components, an internal or lissosphincter of smooth muscle and an outer or external rhabdosphincter of skeletal muscle, that are thought to be responsible for passive and active continence, respectively [4]. The internal sphincter maintains continence during normal activity when there is little stress on the bladder outlet. Its smooth muscle maintains tone for long periods with minimal exertion. The external urethral sphincter is a muscle that is very strong but becomes fatigued very quickly. The action of the external urethral sphincter is often seen when performing cystoscopy after RP and asking the patient to contract. The ability of a patient to circumferentially coapt the urethra on command implies that the muscle tissue and innervations are intact and no hampering fibrosis is present. The two-component model of the urethral sphincter also explains why techniques to spare the bladder neck lead to higher continence rates. Sparing the bladder neck is thought to preserve the majority of the internal sphincter. Preserving this part of the sphincter complex, which is responsible for passive continence, results in earlier return to continence and lower rates of PPI. Multiple studies have shown this to be a contributing factor (LE 3, sample size range 34–240) [5], [6], and [7]. However, Marien and Lepor [41] showed that sparing of the bladder neck has no effect on continence rates (LE 3, sample size 1110).
The supporting structures of the male urethra can be divided into the anterior and posterior support structures and the pelvic floor. The anterior urethral support structures contain the pubourethral ligaments, comprising the pubovesical ligament (PVL), the puboprostatic ligament (PLL), and the tendinous arch of the pelvic fascia. These ligaments stabilize the position of the bladder neck as well as the external sphincter complex, and help to secure the membranous urethra to the pubic bone [8].
The posterior support consists of the perineal body (central perineal tendon), Denonvillier's fascia, the rectourethralis muscle, and the levator ani complex [9] and [10].
The third support structure is the pelvic floor, composed of the levator ani muscle and the surrounding fascia [11]. The pelvic floor is not directly connected to the urethra [11] but plays a role in continence by providing an additional occlusive force on the urethra via increased intra-abdominal pressure [12].
It is postulated that the overall role of the support structures is to provide all-round stability and suspensory support for the urethral sphincter complex [13].
Normally the omega-shaped urethral rhabdosphincter has its anchoring points dorsally at the so-called conjoined fibrous tissue [14]. This conjoined fibrous tissue acts like a fulcrum for the anterior forces exerted by the rhabdosphincter. It compresses the urethra in an anteroposterior direction. If the prostate is dissected, attempts to restore these anchoring properties are generally made to allow proper sphincter functioning [15]. This is usually in the form of puboprostatic ligament–sparing or a stitch to anchor the dorsal venous complex.
It has been shown that preservation of the PPL and PVL to allow proper sphincter functioning improves PPI (LE 1b–3, sample size range 34–691) [6], [15], [16], [17], and [18]. Reconstruction of the posterior musculofascial plate of Denonvilliers or the posterior fibrous raphe, also known as the Rocco stitch seems to improve PPI, as shown by multiple studies (LE 2a–2, sample size range 62–530) [15], [19], and [20], although some surgeons did not note a similar improvement in continence after RP (LE 3, sample size 50) [21]. Total pelvic reconstruction with fixation and anchoring of the sphincteric complex and the bladder urethra anastomosis anteriorly and posteriorly has several biomechanical advantages and also seems to result in earlier return to continence [15]. This technique prevents urethral stump recession, which is also the role of the Rocco stitch (LE 2a, sample size 300) [19]. It also relieves tension on the anastomosis and improves mucosal coaptation at the anastomosis site. Moreover, anterior suspension, by mimicking the PVL and PPL function, alleviates downward prolapse of the bladder on the anastomosis.
There has been much recent debate about the so-called hypermobility of the bulbous urethra that causes PPI and the restoration of continence by correction of this hypermobility and repositioning of the bulbous urethra in males. This is analogous to the hammock theory for women as reported by DeLancey [22].
Burnett and Mostwin [23] observed that upward movement of the sphincter complex occurs when contracting the external urethral sphincter. Based partly on this observation, in those men who do have appropriate sphincter function but have postprostatectomy laxity of the posterior supporting structures, continence may be restored by realignment of the anatomy of the urethral sphincter complex towards the normal, preprostatectomy configuration (LE 2b, sample size 20) [24]. The treatment of PPI with a so-called repositioning and nonobstructive sling is thought to be based on this concept [25] and [26]. However, magnetic resonance imaging (MRI) studies by Suskind et al [27] did not reveal any dislocation of the bulbous urethra after RP (LE 3, sample size 21), placing some doubt that urethral repositioning is the major mechanism of action for the nonobstructing transobturator sling.
A well-performed RP will probably not injure any of the pelvic floor musculature. However, it will change the support for the bladder and may result in varying degrees of incontinence. Direct damage to the puboperinealis muscle and the puboprostatic ligaments can occur after extensive dissection of the prostatic apex or while trying to create a more defined urethral stump. Placing sutures for urethrovesical anastomosis into lateral tissue may also damage the puboperinealis muscle and probably should be avoided; conversely, reconstruction of periprostatic tissue probably leads to better urinary continence outcomes.
Evidence was retrieved from studies with LE 3 and a sample size ranging from 36 to 985 patients. Tuygun et al [28] showed that the incidence of fibrosis is much greater in patients with PPI than in patients without PPI. Consequently, they concluded that fibrosis plays an important role in the development of PPI because it may have a negative effect on external urethral sphincter function. Sacco et al [29] also reported that PPI is more frequent in the case of stricture of the anastomosis when compared to no stricture.
The pudendal nerve supplies innervation to the urethral sphincter complex [30]. The pudendal nerve, according to most anatomists, mainly follows an extrapelvic course via Alcock's canal [30]. Anatomic studies have also shown a partially intrapelvic route for the pudendal nerve branches that go on to innervate the urethral sphincter [31], [32], and [33], and there is some evidence that the neurovascular bundles (NVBs) may, in fact, also play a role in PPI. There is debate about the role and extent of the contribution of the NVB in the innervation of the external urethral rhabdosphincter. Some authors state that it has never been demonstrated that the NVB might contain any somatic nerve supply, and therefore should merely have a functional role in the continence function of the rhabdosphincter [34]. However, others state that it has been demonstrated that the internal urethral sphincter has dense autonomic fibers [35]. Moreover, Strasser and Bartsch [36] found that the NVB directly innervates the membranous urethra. This would mean that damage to the NVB does affect the continence mechanism and preservation does lead to at least earlier recovery of continence after RP. This has been shown by many good-quality studies (LE 2a–3, sample size range 7–536) [5], [29], [37], [38], [39], and [40]. However, it has also been questioned by others who did not see any difference in continence rates between nerve-sparing and non–nerve-sparing techniques (LE 3, sample size 1110) [41].
Newly elucidated anatomic dissections point to the cavernous nerves providing at least a small portion of the innervation to the membranous urethra [42] and [43]. The functional meaning of this is still unclear. However, Nelson et al [44] measured changes in intraurethral pressure caused by intraoperative NVB stimulation and noted a consistent increase in pressure on stimulation in eight consecutive patients. Furthermore, there is ample clinical evidence that NVB preservation during RP leads to earlier recovery of urinary continence (LE 2b, sample size range 42–536) [40] and [45]. This would argue for a direct role of the cavernous nerve/NVBs in the maintenance of urinary continence. The afferent innervation and its effect on subsequent urinary continence are even less well understood [46]. Catarin et al [37] evaluated urethral afferent activity, which appeared to be disrupted after RP and could have an influence on continence after RP (LE 2a, sample size 46).
The urothelium is surrounded by elastic tissue and fibers of smooth and striated muscle. At the junction of the inferior bladder and the proximal urethra, the urothelium becomes a key component of sphincter function. The elastic components of the proximal urethral wall are responsible for coaptation of the urothelium (zone of coaptation). This proper adhesion of the urethral wall provides primary resistance to the urine to maintain continence (LE 3, sample size 985) [29]. Little is known about the optimal length of the zone of coaptation. It is hypothesized that it should be at least 5–10 mm to ensure continence [14].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 66 to 268 patients. The development of de novo post-RP detrusor overactivity (DO) is another biological risk factor that has been suggested to be associated with PPI. Functional changes including DO and reduced compliance may develop after RP due to denervation or devascularization of the urinary bladder [47]. In a study that included 268 patients with LUTS after RP, Lee et al [48] showed that 32.7% of patients with PPI (n = 150) had DO compared to 29.7% of patients without PPI, although this difference was not statistically significant. Song et al [47] observed DO in up to 51% of patients (n = 93) after RP at 3-yr follow-up. However, DO already existed in 27 of the 93 patients (38%) before surgery, and 20 of these 27 (74%) had persistent DO at 3-yr follow-up. The authors attributed the deterioration in storage symptoms among their patients to a reduction in maximum cystometric capacity — possibly involving the absence of urethral-detrusor inhibition — and sphincteric incompetence. In the literature, DO has been reported as the sole cause of PPI in only 4% of cases and was associated with sphincteric incompetence in up to 42% of cases [49].
Evidence was retrieved from studies with LE 3 and a sample size ranging from 111 to 2849 patients. Older patients are expected to have pre-existing LUTS before RP because of an enlarging prostate and/or age-related functional changes in the urinary bladder and urethra. In a study that included 268 patients with LUTS after RP. In a study that included 308 patients who underwent RARP, Novara et al [50] found that patients who recovered their continence 1 yr after surgery were significantly younger than patients in the incontinent group. Karakiewicz et al [51] reported that age was predictive of urinary functional outcomes among 2415 patients after RP. In an analysis of 2849 patients, Matsushita et al [52] confirmed that greater age was an independent predictor of worse continence outcomes at 6 and 12 mo after prostatectomy. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that age was not related to post-RP continence recovery. In addition, Catalona and Basler [54] found no correlation between regaining continence after RP and age in a series of 784 patients.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 111 to 2849 patients. Wolin et al [55] reported that PPI was more prevalent among physically inactive, obese men (BMI >30 kg/m2) in a group of 589 patients with previous RP. Wiltz et al [56] found that among 945 patients who underwent RARP, urinary continence outcomes were significantly lower for patients with BMI >30 kg/m2 at 1- and 2-yr follow-up. Conversely, univariate and multivariate analyses of data for 111 patients by Kadono et al [53] revealed that BMI did not predict the post-RP continence outcome. Hsu et al [57] observed no statistically significant relation between body weight and postoperative continence. In one of the larger series to date to evaluate preoperative predictors of urinary continence, Matsushita et al [52] noted that among 2849 patients who underwent prostatectomy, higher BMI was an independent predictor of worse continence outcomes at 6- and 12-mo follow-up.
Evidence was retrieved from studies with LE 3 for sample sizes ranging from 30 to 124 patients. In a group of 30 patients who underwent TURP before RP, Elder et al [58] found that the PPI incidence was up to 50% among men who underwent TURP 4 wk to 4 mo before RP. The authors recommended waiting 4 mo after TURP before performing RP to decrease the risk of incontinence among such patients. Conversely, in a controlled trial involving 124 patients, Palisaar et al [59] reported no significant difference in PPI rate between patients with and without TURP before RP.
In a study using American Urological Association symptom scores (LE 3, sample size 106), 74 of 106 patients reported occasional leakage after RP; these patients had significantly more noticeable LUTS compared to the 32 patients who were completely dry [60]. Multivariate analysis by Wei et al [61] using data for 482 RP patients revealed that pre-RP baseline continence is a significant predictor of post-RP continence.
Evidence was retrieved from studies with LE 2b–3 for sample sizes ranging from 64 to 2849 patients. Urethral length preservation during RP improves continence outcomes [62]. In patients with a large prostate, RP is theoretically associated with excision of relatively longer parts of the urethra, which might impact continence outcomes in such patients [63]. It has also been assumed that PPI can be explained by pre-existing LUTS among men with a large prostate [57]. In a retrospective analysis of data for 355 patients who underwent extraperitoneal RARP, Boczko et al [64] found that the6-mo post-RP continence rate was significantly lower for men with prostate size >75 cm3 than for those with prostate size <75 cm3. Konety et al [65] reported that patients with prostate size >50 cm3 had lower rates of continence at 6 and 12 mo after RP in a group of 2097 patients. The continence rates equalized across all prostate size categories at 24-mo follow-up. Conversely, univariate and multivariate analyses by Kadono et al [53] using data for 111 patients revealed that prostate size did not predict post-RP continence outcome. In an evaluation of 3067 men, Pettus et al [66] found that prostate size did not appear to have any effect on functional outcomes, including urinary continence, at 12 mo after prostatectomy.
Nguyen et al [67] observed that preoperative urethral sphincter length is approximately 14 mm. Continence recovery at 1 yr was 89% among patients with urethral length >12 mm postoperatively, compared to 77% for patients with urethral length <12 mm. This group also observed that urethral length was significantly associated with quicker postoperative recovery of continence. Longer preoperative and postoperative length was also associated with higher continence rates. Paparel et al [68] evaluated preoperative and postoperative MUL among 64 patients and noted that preoperative and postoperative MUL and the MUL loss ratio were related to recovery time and the level of urinary continence.
On the contrary, Borin et al [69] did not find any difference in continence rates or in the time to achieve continence when dissecting more heavily at the apical borders to achieve less positive surgical margins. Positive apical margins were reduced from to 13% to 5,5% when resecting more of the urethra. The continence rates were similar when the dissection was performed 1 cm below, 0 cm or 1 cm above the urogenital diaphragm [69]. No correlation between continence rates and preoperative or intraoperative urethral stump length was observed. A recent analysis by Matsushita et al [52] evaluating multiple preoperative factors among 2849 men confirmed that longer MUL was strongly associated with improved continence rates at 6 and 12 mo after prostatectomy.
In a systematic review of evidence regarding urinary incontinence after salvage RP, Chade [70] reported continence rates — defined as using no pads — ranging from 21% to 90% for open surgery, from 67% to 78% for laparoscopic surgery, and from 33% to 80% for robotic surgery.
In an attempt to clarify preoperative predictors of urinary continence after prostatectomy, Matsushita [52] included patient age, BMI, and American Society of Anesthesiologists (ASA) score an MUL in the development of a predictive model for PPI. This was a retrospective analysis of data from 2849 patients with RP using multivariate logistic regression. In the base model, univariate analysis revealed that age, BMI, and ASA score were significant independent predictors of continence recovery at 6- and 12-mo follow-up. Membranous urethral length obtained from pre-RP MRI measurements, also an independent predictor, was added to these predictors to develop the final model, which resulted in an increase of 0.085 in AUC for continence recovery at 12 mo over the base model.
Table 1 and Table 2 summarize the factors presumed to cause PPI. Publications both in favor of and contesting these factors are listed to give a clear picture of the current state of the art. Quantitative data are provided in Supplementary Tables 1 and 2.
Table 1
Biological factors contributing to postprostatectomy incontinence
| Factor | Positive | Negative | No effect | Study | LE |
|---|---|---|---|---|---|
| impact | impact | ||||
| Age | * | Novara [50] | 3 | ||
| * | Karakiewicz [51] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| * | Catalona [54] | 3 | |||
| Pre-existing LUTS | * | Rodriguez [60] | 3 | ||
| Functional bladder changes | * | Lee [48] | 3 | ||
| * | Dubbelman [71] | 3 | |||
| * | Song [47] | 2b | |||
| TURP before RP | * | Elder [58] | 3 | ||
| * | Palisaar [59] | 4 | |||
| Prostate size | * | Boczko [64] | 3 | ||
| * | Konety [65] | 3 | |||
| * | Kadono [53] | 3 | |||
| Membranous urethral length | * | Nguyen [67] | 2b | ||
| * | Paparel [68] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Borin [69] | 2b | |||
| * | Hakimi [72] | 3 | |||
| Body mass index | * | Wolin [55] | 3 | ||
| * | Wiltz [56] | 3 | |||
| * | Matsushita [52] | 3 | |||
| * | Kadono [53] | 3 | |||
| Salvage RP after RT | * | Chade [70] |
LE = level of evidence; RP = radical prostatectomy; TURP = transurethral resection of prostate; RT = radiation therapy; LUTS = lower urinary tract symptoms.
Table 2
Surgical factors contributing to postprostatectomy incontinence
| Positive | Negative | No effect | Study | LE | |
|---|---|---|---|---|---|
| impact | impact | ||||
| Fibrosis | * | Paparel [68] | 3 | ||
| * | Tuygun [28] | 3 | |||
| Stricture | * | Sacco [29] | |||
| Extensive dissection | * | Srivastava [73] | 3 | ||
| Bladder neck sparing | * | Stolzenburg [5] | 3 | ||
| * | Selli [7] | 3 | |||
| * | Marien [41] | 4 | |||
| Rocco stitch | * | Rocco [19] | 2a | ||
| * | Kim [21] | 3 | |||
| Anterior fixation | * | Hurtes [16] | 1b | ||
| * | Stolzenburg [17] | 2a | |||
| * | Schlomm [18] | 2b | |||
| * | Soljanik [6] | 3 | |||
| Laxity of the posterior support | * | Bauer [74] | 2b | ||
| * | Rehder [75] | 2b | |||
| * | Suskind [27] | 3 | |||
| Neurovascular bundle damage | * | Montorsi [45] | 2b | ||
| * | Catarin [37] | 2a | |||
| * | Ozdemir [38] | 3 | |||
| * | Kaye [39] | 3 | |||
| * | Sacco [29] | 3 | |||
| * | Stolzenburg [17] | 2a | |||
| * | Burkhard [40] | 2b | |||
| Devascularization | * | Ozdemir [38] | 3 | ||
| * | Myers [76] | 4 | |||
| * | Yucel [77] | 3 |
LE = level of evidence.
There is evidence in the literature that anatomic support and pelvic innervation are important factors in the etiology of PPI. Damage to the urethral sphincter complex, the surrounding structures, or their innervation leads to higher rates of PPI. Certain biological factors and parameters known preoperatively, including greater age, higher BMI, pre-existing LUTS, lower MUL, and functional bladder changes, have a negative impact on continence rates after RP. Among the many surgical and technical factors proposed in the literature as contributing to the development of UI following RP, extensive dissection during surgery, damage to the NVB, and the development of postoperative fibrosis have a substantial negative impact on the continence status of men undergoing RP. Sparing of the bladder neck and anterior, and possibly posterior, fixation of the bladder-urethra anastomosis are associated with better continence rates.
Author contributions: John Heesakkers had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Heesakkers, Stenzl, Farag.
Acquisition of data: Farag, Heesakkers, Bauer, Sandhu, De Ridder.
Analysis and interpretation of data: Heesakkers.
Drafting of the manuscript: Heesakkers, Farag, Bauer, Sandhu, De Ridder.
Critical revision of the manuscript for important intellectual content: Farag.Statistical analysis: None.
Obtaining funding: Heesakkers.
Administrative, technical, or material support: Heesakkers, Farag.
Supervision: Heesakkers, Stenzl.
Other: None.
Financial disclosures: John Heesakkers certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Jaspreet Sandhu has received consultancy fees from American Medical Systems (now Boston Scientific). The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: This manuscript is part of COST Action BM1209 ReST funded by the EU. The sponsor played no direct role in the study.