Background
Short-term trials have demonstrated the efficacy and safety of combination therapy using antimuscarinics and α-blockers in men with lower urinary tract symptoms (LUTS). The Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) II is the first long-term study using solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS).
Objective
To evaluate long-term (up to 52 wk) safety and efficacy of flexible dosing of two fixed-dose combinations (FDC) of Soli plus TOCAS in men with moderate to severe storage symptoms and voiding symptoms.
Design, setting, and participants
Patients with both storage and voiding LUTS, maximum urinary flow rate of 4.0–12.0 ml/s, prostate size <75 ml, and postvoid residuals ≤150 ml, who completed the 12-wk, double-blind NEPTUNE study could continue in the 40-wk, open-label NEPTUNE II study.
Intervention
FDC of Soli 6 mg plus TOCAS 0.4 mg, or Soli 9 mg plus TOCAS 0.4 mg; patients could switch between doses in NEPTUNE II.
Outcome measurements and statistical analysis
Safety and efficacy data from NEPTUNE and NEPTUNE II were combined to cover a 52-wk period. Primary efficacy end points were total International Prostate Symptom Score (IPSS) and total urgency and frequency score (TUFS); secondary end points included IPSS storage and voiding subscores, micturition diary variables, and quality of life parameters.
Results
In all, 1066 men completed NEPTUNE and received one dose or more of study medication in NEPTUNE II. Treatment-emergent adverse events were reported in 499 (46.8%) patients who participated in NEPTUNE II; most were mild or moderate. Urinary retention occurred in 13 of 1208 (1.1%) patients receiving one or more FDCs in NEPTUNE and/or NEPTUNE II; 8 (0.7%) required catheterisation (acute urinary retention [AUR]). Reductions in total IPSS and TUFS during NEPTUNE were maintained for up to 52 wk of FDC treatment, with mean reductions of 9.0 (standard deviation [SD]: 5.7) and 10.1 (SD: 9.2), respectively, from baseline to end of treatment. Clinically relevant improvements were also observed for secondary efficacy end points.
Conclusions
Long-term treatment with FDC Soli plus TOCAS was well tolerated and efficacious in men with storage and voiding LUTS, with a low incidence of AUR.
Patient summary
Treatment with solifenacin plus tamsulosin in a fixed-dose combination tablet was well tolerated by men with lower urinary tract symptoms. Improvements in symptoms were achieved after 4 wk of treatment, with further improvements at week 16 maintained for up to 52 wk throughout the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.
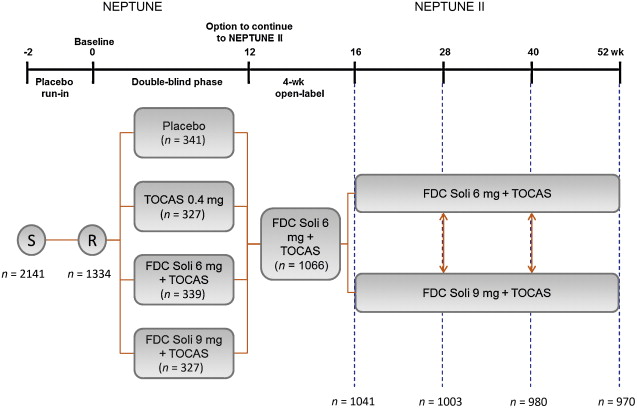
Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.
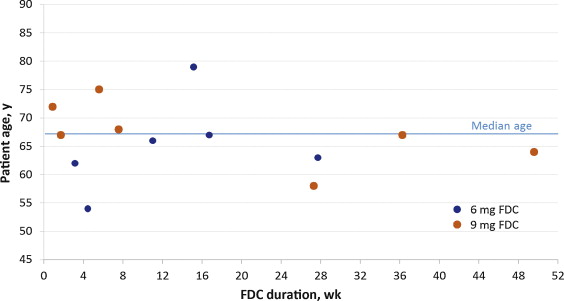
Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).
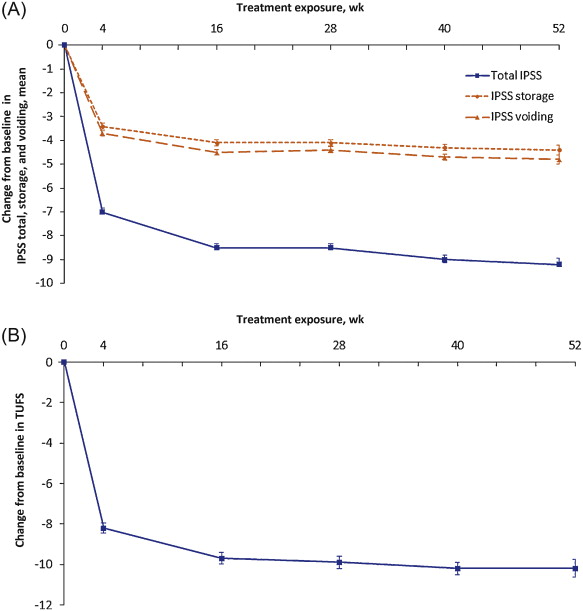
Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).
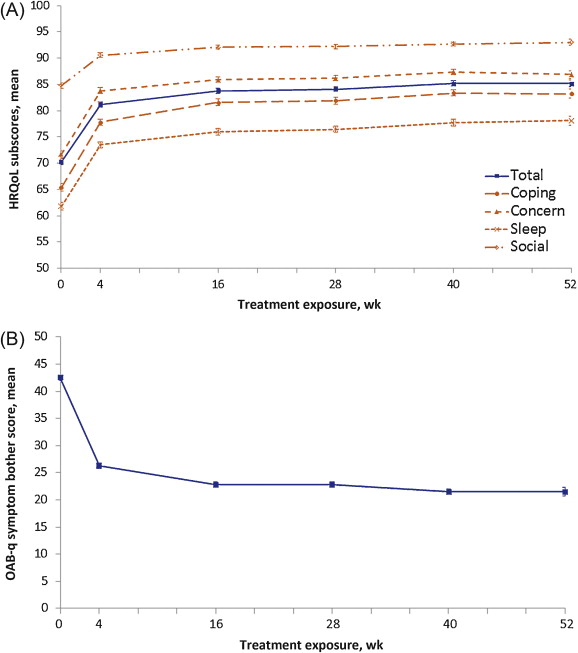
Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
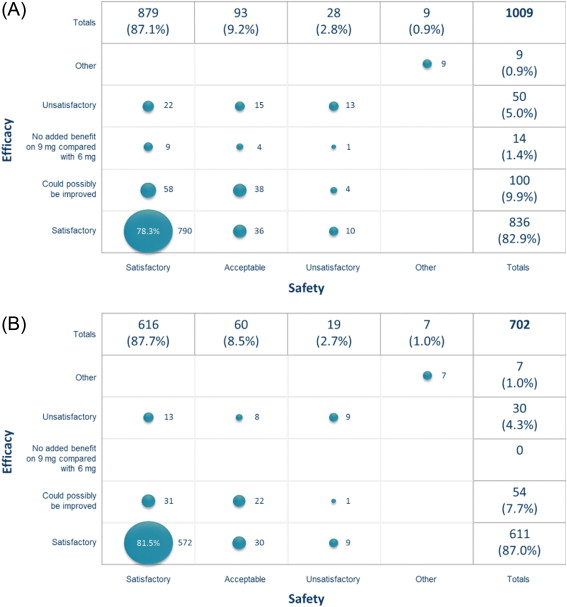
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.
NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.
Lower urinary tract symptoms (LUTS) can be categorised into three groups: storage (eg, urgency, daytime frequency, nocturia), voiding (eg, hesitancy, weak stream, intermittency), and postmicturition (feeling of incomplete bladder emptying and postmicturition dribble)[1] and [2]. These subgroups frequently overlap, with around two-thirds of patients experiencing LUTS from more than one group, and one-third from all three subgroups [3] .
Voiding symptoms are typically managed with α-blockers (α1-adrenoceptor antagonists) [4] , while storage symptoms are usually treated with antimuscarinics [5] . However, storage symptoms, which patients often find more bothersome, are typically undertreated in men, owing to a perceived increased risk of acute urinary retention (AUR) with antimuscarinic agents[6] and [7]. Evidence from clinical trials lasting 4–12 wk with antimuscarinic plus α-blocker combinations suggests that the risk of AUR is low in men with LUTS [8] ; thus, a combination of these two classes of agents appears to be a feasible treatment for men with both storage and voiding LUTS.
Combination therapy with solifenacin (Soli) and the oral controlled absorption system formulation of tamsulosin (TOCAS) has been assessed in clinical studies for the treatment of storage and voiding LUTS. These include two phase 2 studies[9] and [10], in which the two agents were coadministered, and the phase 3 Study of Solifenacin Succinate and Tamsulosin Hydrochloride OCAS (oral controlled absorption system) in Males with Lower Urinary Tract Symptoms (NEPTUNE) study [11] , in which the active ingredients were combined in a once-daily, modified-release, fixed-dose combination (FDC) tablet [11] . In these studies, doses of 6 mg and 9 mg Soli in combination with TOCAS 0.4 mg were well tolerated and offered treatment benefits compared with placebo or TOCAS monotherapy in patients with moderate to severe storage and voiding symptoms.
Patients completing the 12-wk NEPTUNE study were invited to continue into the open-label, 40-wk NEPTUNE II extension study, the only study to date to evaluate the long-term (up to 52 wk in total) safety and efficacy of Soli plus TOCAS combination therapy in male patients with both storage and voiding LUTS.
NEPTUNE II was a multicentre, open-label, flexible-dosing, phase 3 extension study following the 12-week, double-blind NEPTUNE study [11] . Patients received one FDC tablet per day, starting with 4 wk of Soli 6 mg plus TOCAS 0.4 mg (6 mg FDC) followed by either 6 mg FDC or Soli 9 mg plus TOCAS (9 mg FDC) ( Fig. 1 ). Patients could request to switch dosing regimen at any subsequent visit (ie, at 16, 28, and 40 wk from NEPTUNE baseline). The study was approved by independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki and other applicable guidelines, laws, and regulations. Written informed consent was obtained for all patients.

Enrolment into NEPTUNE II required completion of 12 wk of double-blind treatment in NEPTUNE. NEPTUNE enrolled men aged ≥45 yr, with International Prostate Symptom Score (IPSS) ≥13, a maximum urinary flow rate (Qmax) of 4.0–12.0 ml/s, ultrasound-estimated prostate size <75 ml, two or more urgency episodes per 24 h (Patient Perception of Intensity of Urgency Scale [PPIUS] grade 3 or 4) and eight or more micturitions per 24 h. NEPTUNE exclusion criteria included postvoid residuals (PVR) >150 ml, recurrent urinary tract infection, or a history of any other medical condition that, in the investigator's opinion, made the patient unsuitable for inclusion.
Safety parameters were assessed at every visit and included nature and severity of patient-reported adverse events (AEs), PVR volume, Qmax, average urinary flow rate (Qave), vital signs, electrocardiograms (ECGs), and laboratory tests. AUR, defined as urinary retention requiring catheterisation, was considered a serious AE.
Two primary efficacy end points were assessed from baseline (week 0, NEPTUNE) to end of treatment (EoT; up to 52 wk later, NEPTUNE II): total IPSS and Total Urgency and Frequency Score (TUFS). TUFS, previously reported under the name of Total Urgency Score, is a validated measure capturing the two important storage symptoms, urgency and frequency, in a single parameter [12] . TUFS was derived from 3-d micturition diaries grading the level of urgency at each void according to the PPIUS scale (0–4); TUFS was calculated by adding the PPIUS scores of every void in the diary and dividing by the number of days recorded.
Secondary efficacy end points included change from baseline (NEPTUNE) to EoT in IPSS storage and voiding subscores, micturition diary variables (micturition frequency, urgency, incontinence episodes, voided volume) and quality of life (QoL) parameters (IPSS QoL score and overactive bladder questionnaire [OAB-q]). Additionally, at each visit, patients were asked to rate the safety and efficacy of the dose for the preceding treatment period when selecting the dose for the next period.
Safety and efficacy data from patients taking part in both NEPTUNE and NEPTUNE II were combined for both FDCs, covering a 52-wk period in total. Data were analysed by duration of exposure to FDC (4, 16, 28, 40, and 52 wk). Total FDC therapy was up to 40 wk for patients receiving placebo or TOCAS monotherapy in NEPTUNE, and up to 52 wk for those receiving either FDC in NEPTUNE. Efficacy analyses were performed on the full analysis set (FAS): all patients who received one or more doses of study medication during NEPTUNE II and had a total IPSS value at baseline plus at least one postdose value during NEPTUNE II, or a TUFS value at baseline plus at least one postdose value during NEPTUNE II. Safety analyses were performed on the safety analysis set: all patients who received one or more doses of study medication during NEPTUNE II. Only descriptive statistics have been reported for safety and efficacy variables.
Of 1199 patients completing the NEPTUNE study, 1067 were enrolled in NEPTUNE II and 1066 (88.9%) received one or more doses of study medication. In total, 106 patients (9.9%) discontinued prematurely, mainly due to AEs (n = 43; 4.0%), withdrawal of consent (n = 23; 2.2%), or lack of efficacy (n = 19; 1.8%). Patient baseline characteristics are summarised in Table 1 .
Table 1 Patient baseline characteristics for NEPTUNE II *
| Characteristics | Soli plus TOCAS FDC (n = 1066) |
|---|---|
| Age, yr | 65.1 (8.11; 45–86) |
| Race, no. (%) | |
| White | 1060 (99.4) |
| Black | 3 (0.3) |
| Asian | 2 (0.2) |
| Arab | 1 (0.1) |
| BMI, kg/m2 | 28.4 (4.2; 19–55) |
| Estimated prostate size, ml | 37.8 (13.9; 9–74) |
| PVR, ml, median (range) | 26.0 (0–150) |
| PSA level, ng/ml | 2.22 (2.02; 0.1–15.6) |
| Qmax, ml/s | 8.9 (1.9; 4.0–12.0) |
| Qave, ml/s | 4.9 (1.4; 1.0–8.8) |
* Visit 2, safety analysis set. Baseline for NEPTUNE II is the start of the 12-wk NEPTUNE study.
BMI = body mass index; FDC = fixed-dose combination; PSA = prostate-specific antigen; PVR = postvoid residual; Qave = average urinary flow rate; Qmax = maximum urinary flow rate; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Data are given as mean (standard deviation; range) unless otherwise indicated.
All patients participating in NEPTUNE II initially received 6 mg FDC for 4 wk, from visit 5 to visit 6. At visit 6, 241 patients (23.2%) elected to increase to 9 mg FDC, primarily with the aim of improving efficacy, with fewer patients choosing to increase dose at subsequent visits ( Table 2 ). At visit 7, 49 (21.4%) patients who increased dose at visit 6 chose to return to the 6 mg FDC, primarily because of insufficient additional benefit. At the final visit, 681 (70.2%) of 970 patients would have chosen 6 mg FDC and 289 (29.8%) would have chosen 9 mg FDC if the study had continued.
Table 2 Elective dose changes during the open-label NEPTUNE II study
| Visit | No. of patients | Dose increase, no. (%) | Dose decrease, no. (%) | Total 6 mg Soli plus TOCAS FDC, no. | Total 9 mg Soli plus TOCAS FDC, no. |
|---|---|---|---|---|---|
| 5 | 1066 | NA | NA | 1066 | 0 |
| 6 | 1041 | 241 (23.2) | NA | 800 | 241 |
| 7 | 1003 | 99 (12.8) | 49 (21.4) | 724 | 279 |
| 8 | 980 | 57 (8.1) | 33 (12.1) | 683 | 297 |
| 9 | 970 | 21 (3.1) | 24 (8.2) | 681 | 289 |
FDC = fixed-dose combination; N/A = not applicable; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system.
Of 1066 patients receiving treatment in NEPTUNE II, 499 (46.8%) experienced treatment-emergent adverse events (TEAEs) while on FDC in NEPTUNE or NEPTUNE II; the majority were mild to moderate in severity. The most common were dry mouth (12.4%), constipation (5.2%), and dyspepsia (2.7%) ( Table 3 ). A total of 86 serious AEs (SAEs) were reported by 64 (6.0%) patients, 12 of whom had events considered possibly or probably related to the study drug. Only two SAEs occurred in two or more patients: three cases of intervertebral disc protrusion and six cases of AUR (four considered possibly or probably related to treatment). There were three deaths (0.3%) during NEPTUNE II; none considered related to treatment (one patient experienced depression and committed suicide, one died of myocardial infarction, and one suffered a cerebrovascular accident).
Table 3 Summary of treatment-emergent adverse events in patients receiving treatment in NEPTUNE II, occurring while on fixed-dose combinations in NEPTUNE or NEPTUNE II (safety analysis set)
| Patients | Soli plus TOCAS FDC (n = 1066), no. (%) |
|---|---|
| TEAEs | 499 (46.8) |
| Mild | 300 (28.1) |
| Moderate | 159 (14.9) |
| Severe | 40 (3.8) |
| Drug-related AEs | 255 (23.9) |
| Mild | 165 (15.5) |
| Moderate | 72 (6.8) |
| Severe | 18 (1.7) |
| Serious AEs | 64 (6.0) |
| Total serious AEs, no. | 86 |
| Drug-related serious AEs | 12 (1.1) |
| Total drug-related serious AEs, no. | 12 |
| Discontinued owing to AEs | 42 (3.9) |
| Discontinued owing to drug-related AEs | 28 (2.6) |
| Deaths | 3 (0.3) |
| Drug-related deaths | 0 |
| Most frequent TEAEs (≥1% patients) | |
| Dry mouth | 132 (12.4) |
| Constipation | 55 (5.2) |
| Dyspepsia | 29 (2.7) |
| Hypertension | 26 (2.4) |
| Urinary tract infection | 24 (2.3) |
| Back pain | 21 (2.0) |
| Bronchitis | 12 (1.1) |
| Erectile dysfunction * | 11 (1.0) |
| Nasopharyngitis | 11 (1.0) |
* Erectile dysfunction occurred in 11 patients (1.0%), while retrograde ejaculation and ejaculation failure, classified separately, occurred in another 7 patients (0.7%) and 2 patients (0.2%), respectively.
FDC = fixed-dose combination; Soli = solifenacin; TEAE = treatment-emergent adverse event; TOCAS = tamsulosin oral controlled absorption system.
Data given as number (percentage) unless otherwise indicated.
At baseline, all patients had a PVR ≤150 ml, as defined by study inclusion criteria. Median PVR increased from 26.0 ml (range: 0–150 ml) at baseline to 32.0 ml (range: 0–346 ml) at EoT. Sixty-eight patients (6.4%) developed a PVR of ≥150 ml at any time during the studies, including 4 (0.4%) ≥300 ml. Four of these 68 patients developed AUR.
Of all patients who received FDC during NEPTUNE and/or NEPTUNE II, including those who did not continue into NEPTUNE II, 13 of 1208 (1.1%) developed urinary retention, 8 of these 13 (0.7%) had AUR. The duration of FDC treatment preceding onset of urinary retention ranged from 6 d to 347 d (median: 77 d) ( Fig. 2 ). Episodes occurred within the first 16 weeks of treatment in 8 of 13 (61.5%) patients.

Fig. 2 Duration of fixed-dose combination (FDC) until onset of urinary retention, by age and FDC dose.
Qmaxand Qaveincreased from baseline to EoT by 4.5 ml/s (from 8.9 ml/s to 13.5 ml/s) and 2.1 ml/s (from 4.9 ml/s to 6.9 ml/s), respectively. There were no relevant changes in vital signs, ECG parameters, physical examinations, or standard laboratory measurements. The incidence of TEAEs based on abnormal laboratory test results was ≤1.0%.
Reductions in both mean total IPSS and TUFS observed in the NEPTUNE study with FDC treatment were maintained throughout NEPTUNE II. Mean total IPSS was reduced by 9.0 points (standard deviation [SD]: 5.7), from 18.7 (SD: 4.4) at baseline to 9.7 (SD: 5.9) at EoT, equivalent to a 48.1% reduction. Mean TUFS was reduced by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), a 37.1% reduction ( Fig. 3 ; Table 4 ).

Fig. 3 Mean (plus or minus the standard error) of changes from baseline to end of treatment: (A) International Prostate Symptom Score (IPSS) total, storage, and voiding; (B) total urgency and frequency score (TUFS). Decreases in IPSS, IPSS subscores, or TUFS indicate improvement. Total IPSS comprised seven LUTS questions, three for evaluation of the storage subscore (IPSS 2 + 4 + 7) and four for the voiding subscore (IPSS 1 + 3 + 5 + 6). Graphs are based on descriptive statistics.
Table 4 Changes in efficacy variables from baseline in NEPTUNE to end of treatment in NEPTUNE II (safety analysis set)
| Changes | Soli plus TOCAS FDC |
|---|---|
| Total IPSS | n = 1004 |
| Baseline | 18.7 (4.4) |
| End of treatment | 9.7 (5.9) |
| Change | −9.0 (5.7) |
| TUFS | n = 1005 |
| Baseline | 27.2 (8.6) |
| End of treatment | 17.1 (9.5) |
| Change | −10.1 (9.2) |
| IPSS storage subscore | n = 1004 |
| Baseline | 8.9 (2.4) |
| End of treatment | 4.6 (2.7) |
| Change | −4.3 (2.9) |
| IPSS voiding subscore | n = 1004 |
| Baseline | 9.8 (3.6) |
| End of treatment | 5.2 (4.1) |
| Change | −4.7 (4.1) |
| Micturitions per 24 h, mean | n = 1008 |
| Baseline | 11.4 (2.6) |
| End of treatment | 8.9 (2.6) |
| Change | −2.5 (2.4) |
| Urgency episodes per 24 h * , mean | n = 1006 |
| Baseline | 5.4 (3.2) |
| End of treatment | 2.3 (3.3) |
| Change | −3.1 (3.5) |
| Incontinence episodes per 24 h ** , mean | n = 250 |
| Baseline | 1.8 (2.0) |
| End of treatment | 0.4 (1.5) |
| Change | −1.4 (2.1) |
| Voided volume per micturition, ml, mean | n = 1008 |
| Baseline | 162.8 (47.6) |
| End of treatment | 201.8 (68.5) |
| Change | 39.0 (47.3) |
* Patient Perception of Intensity of Urgency Scale grade 3–4.
** Patients with at least one episode at baseline.
FDC = fixed-dose combination; IPSS = International Prostate Symptom Score; Soli = solifenacin; TOCAS = tamsulosin oral controlled absorption system; TUFS = Total Urgency and Frequency Score.
Data are mean (standard deviation) unless otherwise indicated.
Mean IPSS storage and voiding subscores were reduced from baseline to EoT by 4.3 (SD: 2.9) and 4.7 (SD: 4.1) points, respectively ( Fig. 3 ; Table 4 ). These changes are equivalent to a 48.3% improvement in storage symptoms and 48.0% improvement in voiding symptoms.
Improvements in micturition diary variables were evident at the first time point of 4 wk of FDC treatment, with further improvements reported at week 16 that were maintained throughout NEPTUNE II. The mean number of micturitions, urgency episodes, and incontinence episodes per 24 h were reduced by 2.5 (SD: 2.4), 3.1 (SD: 3.5), and 1.4 (SD: 2.1), respectively, from baseline to EoT, equivalent to reductions of 21.9%, 57.4%, and 77.8%, respectively, while mean voided volume per micturition increased by 39.0 ml (SD: 47.3), a 24.0% increase ( Table 4 ).
The mean IPSS QoL score was reduced by 1.9 points (SD: 1.5), from 4.1 (SD: 1.1) at baseline (mostly dissatisfied) to 2.1 (SD: 1.4) at EoT (mostly satisfied), equivalent to a 46.3% improvement. Of the 1003 patients providing EoT data for this question, 856 (85.3%) scored ≤3 at EoT, compared with 317 (31.6%) at baseline. QoL parameters, as assessed by OAB-q, improved with FDC treatment. Mean symptom bother score was reduced from baseline to EoT by 20.6, and increases in mean health-related QoL total score and subscores were seen. Improvements were already documented at 4 wk, with further improvements at 16 wk that were maintained to EoT ( Fig. 4 ).

Fig. 4 Mean (plus or minus the standard error) health-related quality of life (HRQoL) scores by treatment duration for (A) overactive bladder questionnaire (OAB-q) total and subscores and (B) OAB-q symptom bother scores. An increase in the domains of the OAB-q and a decrease in the OAB-q bother score indicate improvement of HRQoL related to bother. Graphs are based on descriptive statistics.
Considering the dose taken in the period prior to their end-of-study visit, 87.1% of patients were satisfied with its safety, 82.9% were satisfied with its efficacy, and 78.3% were satisfied with both safety and efficacy ( Fig. 5 A). Of those ending the study on 6 mg FDC, 87.7% were satisfied with its safety and 87.0% were satisfied with its efficacy ( Fig. 5 B), versus 85.8% and 73.3%, respectively, for those ending the study on 9 mg FDC. When asked which dose they would have taken had the study continued, 681 (70.2%) indicated 6 mg FDC and 289 (29.8%) indicated 9 mg FDC.

NEPTUNE II is currently the only reported study to evaluate the long-term safety and efficacy of combination therapy with an antimuscarinic and an α1-blocker in men.
In the NEPTUNE and NEPTUNE II studies, long-term treatment with FDC Soli plus TOCAS for up to 52 wk was well tolerated and offered clinically relevant treatment benefits that were sustained until the EoT. The frequency of TEAEs was low and in line with prior experience of Soli and tamsulosin monotherapies. Our results confirm those of the 12-wk, phase 2 solifenacin and tamsulosin in males with lower urinary tract symptoms associated with benign prostatic hyperplasia (SATURN) study [10] , in which Soli plus TOCAS combination therapy was well tolerated and associated with significant efficacy and QoL benefits versus TOCAS monotherapy in the subgroup of patients with moderate to severe storage symptoms and voiding symptoms.
Historically, the use of antimuscarinics in men with LUTS has been limited, owing to concerns about the development of AUR or increases in PVR [2] . In NEPTUNE and NEPTUNE II, urinary retention was experienced by only 13 (1.1%) patients receiving FDC, 8 (0.7%) of whom experienced AUR. This is similar to the AUR rate observed with combination therapy in the 12-wk SATURN study (0.4%) [10] . The NEPTUNE study excluded patients with baseline PVR >150 ml, compared with >200 ml in SATURN, but additional research is required to determine whether the risk of AUR is higher with increased PVR. Although baseline prostate size in NEPTUNE was restricted to <75 ml, the study included men with enlarged prostates. Median prostate size was 36 ml, thus 50% of patients had a baseline prostate size between 36 ml and 74 ml. Enlarged prostates and high symptom scores, observed in this study at baseline, are known risk factors for AUR [13] ; therefore, patients may be considered at high risk of AUR, despite the PVR restriction. Further study in men with high PVR volume or large prostate size may provide additional relevant information for prescribers. Counselling with regard to the signs and symptoms of urinary retention and monitoring of PVR should be considered for potentially at-risk patients prescribed combination therapy.
Over the 52-wk period of NEPTUNE and NEPTUNE II, total IPSS was reduced by 9.0 points, considerably greater than the average 3.0-point decrease considered to indicate slight improvement, and matching the 8.8-point decrease indicating marked symptomatic improvement [14] . Considerable reductions in TUFS from baseline to EoT by 10.1 points (SD: 9.2), from 27.2 (SD: 8.6) to 17.1 (SD: 9.5), were also achieved with treatment of up to 52 wk, confirming sustained improvements observed in both urinary urgency and frequency. Since TUFS correlates with patient satisfaction and QoL assessments [15] , these improvements could be drivers of patient satisfaction. Additionally, volume voided per micturition was increased, which may partly explain the increase in Qmax.
A potential limitation of the NEPTUNE II study is that no placebo arm was included. Consequently, the percentage of patients reaching a clinically relevant improvement on FDC treatment in NEPTUNE II could not be compared with a control arm. However, clinically relevant effect sizes were maintained from the 12-wk NEPTUNE study, in which significant benefit was demonstrated for all key efficacy parameters over placebo and for storage and QoL parameters over tamsulosin. In addition, NEPTUNE II did not allow prospective comparison of the 6 mg and 9 mg FDCs, as patients were allowed to switch doses based on their experience in the study. The analyses, therefore, combine data from the two dose groups. However, this limitation also provides interesting information on patients’ treatment decisions. Reasons for dose increases were mostly related to a potential increase in efficacy, while the main reason for a dose decrease was insufficient efficacy benefit with the higher dose. Most patients indicated that they would have chosen the 6 mg FDC if the study had continued. Based on the results obtained in the NEPTUNE and NEPTUNE II studies, the FDC of Soli 6 mg and TOCAS 0.4 mg has been registered in several European countries, while registration procedures are ongoing in other countries.
The FDC of Soli and TOCAS 0.4 mg was efficacious and well tolerated during long-term therapy for up to 52 wk in the population studied. The incidence of AUR was low (0.7%), indicating this combination therapy can offer clinically relevant benefits in men with moderate to severe storage symptoms and voiding symptoms.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Klaver, Drogendijk, Drake, Van Kerrebroeck.
Acquisition of data: Chapple, Drake, Van Kerrebroeck.
Analysis and interpretation of data: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Drafting of the manuscript: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Sokol, Oelke, Traudtner, Klaver, Drogendijk, Van Kerrebroeck.
Statistical analysis: Drogendijk.
Obtaining funding: none.
Administrative, technical, or material support: None.
Supervision: Drake.
Other(specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M. Drake: advisory board member, speaker, and researcher for Allergan, Astellas, Ferring; consultant for Apogepha; researcher for Coloplast. C. Chapple: consultant for AMS, Lily, ONO; consultant, researcher, speaker, and trial participation for Allergan, Astellas, Pfizer, and Recordati. M. Oelke: consultant, speaker, and/or trial participant for Apogepha Arzneimittel, Astellas, Biocompatibles, GlaxoSmithKline, Lilly, Munipharma, Pfizer, Sophiris, and Recordati; research grant recipient from Astellas and Pfzer. K. Trautdner, M. Klaver, and T. Drogendijk are employees of Astellas Pharma. P. Van Kerrebroeck: lecturer for Astellas, Ferring, and Medtronic; advisory board member for Allergan, Astellas, Ferring, and Medtronic.
Funding/Support and role of the sponsor: This study was supported by Astellas Pharma, which was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript. Medical writing and editorial assistance for the preparation of this manuscript were funded by Astellas.
Acknowledgement statement: The authors would like to thank all of the patients, study centres, and investigators who took part in the NEPTUNE and NEPTUNE II trials. The trials were conceived and funded by Astellas Pharma Europe B.V. Medical writing and editorial assistance for the preparation of this manuscript were provided by Derek Lavery, Caroline Loat, and Lindsay Napier at Darwin Healthcare Communications, UK. The authors would like to thank Dominique Bongaerts and Annemarie Voortman for their contributions to the execution of the study.