Context
Several preclinical reports, randomized controlled trials, systematic reviews, and posthoc analyses corroborate the role of phosphodiesterase type 5 inhibitors (PDE5-Is) in the treatment of men with lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE).
Objective
Update of the latest evidence on the mechanisms of action, evaluate the current meta-analyses, and emphasize the results of pooled data analyses of PDE5-Is in LUTS/BPE.
Evidence acquisition
Literature analysis of basic researches on PDE5-Is, systematic literature search in PubMed and Scopus until May 2015 on reviews of trials on PDE5-Is, and collection of pooled data available on tadalafil 5 mg.
Evidence synthesis
Latest evidences on the pathophysiology of LUTS/BPE has provided the rationale for use of PDE5-Is: (1) improvement of LUT oxygenation, (2) smooth muscle relaxation, (3) negative regulation of proliferation and transdifferentiation of LUT stroma, (4) reduction of bladder afferent nerve activity, and (5) down-regulation of prostate inflammation are the proven mechanisms of action of PDE5-Is. Data from eight systematic reviews demonstrated that PDE5-Is allow to improve LUTS (International Prostate Symptom Score mean difference vs placebo: 2.35–4.21) and erectile function (International Index of Erectile Function mean difference vs placebo: 2.25–5.66), with negligible change in flow rate (Qmax mean difference vs placebo: 0.01–1.43). Pooled data analyses revealed that tadalafil 5 mg once daily allows the clinically-meaningful improvement of LUTS and nocturnal voiding frequency independent of both erectile dysfunction severity and improvement.
Conclusions
PDE5-Is are safe and effective in improving both LUTS and erectile function in appropriately selected men with LUTS/BPE. Data on the reduction of disease progression, long-term outcomes, and cost-effectiveness analyses are still lacking.
Patient summary
We reviewed recent literature on phosphodiesterase type 5 inhibitors in men with lower urinary tract symptoms associated with prostatic enlargement. We found evidence to confirm that phosphodiesterase type 5 inhibitors are a valid treatment option for men affected by bothersome urinary symptoms with or without erectile dysfunction.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).
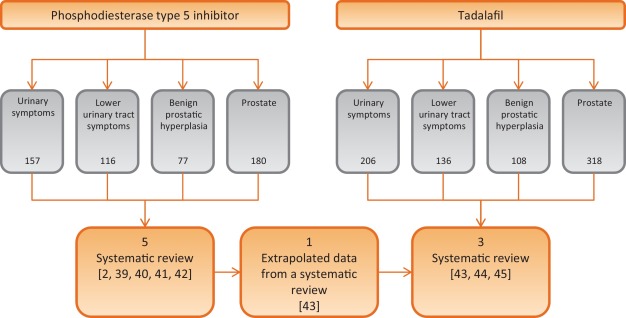
Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) associated with benign prostatic enlargement (BPE) and erectile dysfunction (ED) are both highly prevalent in elderly men [1]. Although the underlying pathophysiological links between LUTS and ED are not completely understood, both conditions are amenable to therapy with phosphodiesterase type 5 inhibitors (PDE5-Is) [2]. Recently, several studies have suggested that metabolic factors could be important for contributing to both prostate inflammation and enlargement in men with LUTS [3] and [4]. It has been hypothesized that PDE5-Is could reduce inflammation with the associated fibrosis and improve the oxygenation of the human prostate, with a normalization of prostatic structural anatomy and physiologic activity [5] and [6].
The aim of this review is to provide an update on the current knowledge on the potential mechanisms of action of PDE5-Is on LUTS/BPE, critically analyze systematic reviews and relevant data from the current literature on the clinical use of all PDE5-Is, and report the latest evidence on pooled-data analyses of tadalafil once daily to provide a definite rationale for the clinical use of tadalafil for the treatment of LUTS/BPE.
The pathways mediating the activity of PDE5-Is: LUTS have a multifactorial pathophysiology and have been discussed extensively [7], [8], and [9]. Traditionally, the prostate and urethra has been the focus in the pathogenesis of male LUTS. Chalfin and Bradley [10] blocked prostate sensory afferents with lidocaine in patients with detrusor overactivity (DO) and found that overactivity was eliminated in 10/11 patients, but had no effect in four patients with a normal cystometry. Based on these findings, they suggested that sensory stimuli from an anatomically altered prostatic urethra induced DO, and that permanent ablation of sensory stimuli from the prostate in patients with outlet obstruction would be of benefit.
The role of the bladder in male LUTS has been emphasized [9], and much attention has been given to the involvement of the different components of the bladder wall. Changes of the urothelium, afferent nerves, lamina propria, vasculature, and detrusor smooth muscle may initiate LUTS and thus emerge as therapeutic targets [11]. Most probably all of these components are more or less simultaneously involved in the afferent signaling resulting in LUTS, but at least two distinct signaling pathways can be identified: the urothelial and the myogenic pathway [12]. The urothelial pathway is a functional unit consisting of the urothelium, interstitial cells, and afferent nerves (C-fibers) in the lamina propria. The myogenic pathway is activated via in-series mechanoreceptors responding to distention, and via spontaneous contractile activity in units of myocytes generating afferent noise. Urothelial cells have the ability to sense changes in their extracellular environment, and respond to chemical, mechanical, and thermal stimuli by releasing various factors such as adenosine triphosphate, nitric oxide (NO), and acetylcholine. The bladder develops tone during filling and also exhibits nonsynchronized local contractions and relaxations that are caused by a basal myogenic mechanical activity that may be reinforced by the release of acetylcholine from non-neuronal and/or neuronal sources or local mediators, such as prostaglandins and endothelins. It has been suggested that these spontaneous contractions are able to generate activity in afferent nerves that may contribute to LUTS [13]. The central nervous system may be an important initiator of LUTS [14] and [15]. According to Sakakibara et al [15] there is compelling evidence to suggest that the central nervous system's white matter disease can cause an overactive bladder and incontinence, and in some patients these might be the initial manifestation.
In the pathophysiology of LUTS, the NO/cyclic guanosine monophosphate (cGMP) (NO/cGMP) signaling pathway is believed to play a central role. Since NO signaling occurs via stimulation of soluble guanylate cyclase producing cGMP, it is reasonable to assume that the level of cGMP in different LUT tissues can influence their ability to generate LUTS. One way of modulating cGMP levels is to selectively inhibit cGMP degradation, and it is generally believed that all effects of PDE5Is are mediated by selective inhibition of the degradation of cyclic GMP.
Mechanism of action of PDE5-Is: In several animal species, including humans, the entire LUT expresses PDE5, with a relatively higher abundance within the bladder [16] and [17]. In human bladder homogenates, vardenafil, sildenafil, and tadalafil inhibited PDE5 activity with IC50s strictly related to those measured in human penile tissue, that is 0.3 nmoles/L, 3 nmoles/L, and 6 nmoles/L, respectively [16].
Although it is well established that PDE5 is expressed and biologically active in LUT, its functional role is still a matter of debate. Clinical studies demonstrated that its inhibition, through all the aforementioned PDE5-Is, resulted in amelioration of LUTS. Several mechanisms of action of PDE5-Is in LUTS have been hypothesized.
Immunohistochemical studies indicate that PDE5 is localized in the endothelial and smooth muscle cells of the LUT blood vessels [17]. In particular, in the human vesicular-deferential artery PDE5 mRNA is higher than in the bladder and as abundant as in corpora cavernosa extracts. In this vessel, PDE5 enzymatic activity was inhibited by tadalafil incubation [19]. In isolated human vesicular-deferential artery rings, tadalafil was able to increase the vasorelaxant response to a NO donor, suggesting an active role of PDE5 in controlling LUT perfusion. In a genetic rat model of OAB and prostate alteration due to LUT ischemia, short-term administration of vardenafil and tadalafil increases bladder and prostate oxygenation [22] and [23]. Tadalafil effect was even higher after prolonged in vivo administration [22]. A tadalafil-induced decrease in hypoxia-related genes was also reported in a rabbit bladder [24]. Recently, in other rat models of ischemia/perfusion or of partial bladder outlet obstruction, tadalafil was reported to improve impaired prostate and/or bladder perfusion [20] and [21]. Bartolotto et al [25], observed hemodynamic changes in the prostate of 12 men with BPH after only 90 min of tadalafil administration, with using contrast-enhanced ultrasound. However, in a larger trial involving 97 patients with BPH-LUTS, once daily tadalafil for 8 wk did not result in changes in hemodynamic parameters in the prostate or bladder neck, as detected with transrectal ultrasound [26].
Several in vitro studies have demonstrated that PDE5-Is can relax isolated prostate and bladder neck strips [27], [28], and [29], most probably by increasing NO signaling from nerve fibers present in close proximity to the muscular structures of the LUT. Because of the lower nitrergic representation, the bladder dome was more resistant to the relaxing effect of PDE5-Is [28]. The PDE5-Is relaxant effect is more evident in preparation of bladder neck and prostate primed with a NO donor (sodium nitroprusside [SNP]), than in naïve ones [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], and [29]. In SNP-primed human prostate strips, tadalafil was able to increase cGMP content in a concentration-dependent fashion [29]. By blocking cGMP formation through an inhibitor of soluble guanylate cyclase, there was a significant decrease in sildenafil-induced relaxation of human bladder dome [28]. In the same study, it was also demonstrated that other signaling pathways are involved in mediating sildenafil relaxant action, like cAMP and potassium-dependent channels [28]. However, all these effects were obtained at high sildenafil concentrations. It is possible that part of the relaxing effects of PDE5 inhibitors in the bladder are mediated by a cGMP-dependent, cGMP dependent protein kinase-mediated, phosphorylation of the small GTPase RhoA on Ser188, thereby inhibiting its membrane translocation and the RhoA ability to activate its downstream kinase, ROCK [22]. An overactivity of the RhoA/ROCK signaling has been proposed as one of the biological determinants of LUTS. In a diet-induced bladder dysfunction model in rabbit, tadalafil dosing for one week decreased RhoA/Rho-associated protein kinase (ROCK) signaling and normalized “in vitro” hyper-sensitivity to the ROCK inhibitor Y-27632 [20]. In rat models of partial urethral obstruction vardenafil [23] and [30] and tadalafil [21] and [31] were able to ameliorate urodynamic signs of bladder outlet obstruction.
In the human prostate [17] and [33], bladder [16] and [17], and urethra [17] preparations, PDE5 was virtually absent in the epithelial cells and expressed only in stromal cells, beside blood vessels. In the human prostatic stroma, PDE5 is colocalized with its main substrate cGMP and its downstream kinases [27]. Nanomolar concentrations of vardenafil increase SNP-induced antiproliferative effects, with an effect that was more evident in the bladder and urethra [17], mediated by the cGMP-PKG pathway [23] and [33]. Transforming growth factor-β induced fibroblast–myofibroblast transdifferentiation in human prostatic cells [33] and explants [32] were inhibited by tadalafil and vardenafil, as well as by PDE5 knockdown. Vardenafil was even able to reverse stromal remodeling, a central program underlying BPH progression [32]. Accordingly, in a rat model of chronic ischemia, prostatic stroma overgrowth was attenuated by tadalafil dosing [34]. Similar results were previously reported in a rabbit prostate [26]. However, in some experimental models, chronic tadalafil administration did not result in a decreased prostate weight [24] and [34]. Tadalafil administration in rat models was associated with bladder antiproliferative [21] and [31] and antifibrotic [31] effects. Several genes related to myofibroblast differentiation and fibrosis were down-regulated in the bladder extracts or cells after tadalafil administration [24]. In addition, in human and rabbit bladder myofibroblasts, endothelin-1 or platelet-derived growth factor-induced cell migration was inhibited by vardenafil and tadalafil, respectively, in a PKG-dependent way [22], [23], and [24].
The NO/cGMP signaling pathway is likely to have a significant role in the innervation of the LUT. In human, neuronal NO synthase and guanylate cyclase are expressed in the uroepithelium, in neural fibers of the bladder neck and prostatic urethra, and in afferent nerves and interstitial cells, respectively [35]. In rats PDE5-Is may decrease the activity of afferent nerves in the LUT via an activation of the NO/cGMP pathway and thereby possibly decreasing the perception of bladder filling and reducing the sensation of urgency. Cystometry experiments in rat models of distended or irritated bladder suggested that pharmacological recruitment of the NO/cGMP pathway, especially with PDE5-Is, exerted an inhibitory effect on bladder afferent activity [36]. In a controlled pilot clinical study in spinal cord injury patients with neurogenic DO, acute administration of vardenafil significantly improved urodynamic parameters [37].
Emerging evidences indicate that metabolic derangements, clustered in the metabolic syndrome (MetS) construct, may have a role in BPH pathophysiology. Within MetS, visceral obesity and dyslipidemia are the major predictors of an enlarged prostate size [3]. In a rabbit model of MetS-associated prostate alterations tadalafil dosing was able to reduce prostate inflammation and leukocyte infiltration, along with hypo-oxygenation and fibrosis/myofibroblast activation [6]. In isolated human BPH stromal cells, both tadalafil and vardenafil decreased tumor necrosis factor α-induced expression of genes related to inflammation and tissue remodeling. Similar results were obtained when tumor necrosis factor α-induced secretion of interleukin-8 and interferon γ-induced protein 10 was considered. Both vardenafil and tadalafil were able to blunt interleukin-8 secretion induced also by metabolic stimuli. Finally, both PDE5-Is significantly reduced the ability to increase the expression of oxidized low density lipoprotein receptor, lectin-like oxidized low-density lipoprotein receptor-1 [5].
A systematic literature search in PubMed and Scopus was performed until May 2015, to identify systematic reviews of randomized controlled trial (RCTs), including the following MeSH terms: phosphodiesterase type 5 inhibitor and tadalafil PLUS urinary symptoms OR lower urinary tract symptoms OR benign prostatic hyperplasia OR prostate. We included only systematic reviews of RCTs comparing PDE5-Is treatment with different oral therapies or placebos for LUTS/BPH, while we excluded reviews of RTCs without PDE5-Is, nonclinical trials with PDE5-Is, or nonsystematic review. A total of eight papers were analyzed (Fig. 1; Table 1).

Table 1 Characteristics of the studies included in the review
| Study included | Outcomes | Notes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | No. of RCTs | No. P.ts | Drugs | Duration (wk) |
Jadad Score |
IPSS | IIEF | Qmax | ||||
| S | V | T | O | Mean diff. vs placebo | Mean diff. vs placebo | Mean diff. vs placebo | ||||||
| Laydner et al [39] | 4 | 1928 | 1 | 1 | 2 | – | 12 | 3–5 | – | – | – | First systematic review in literature without meta-analysis |
| Liu et al [40] | 11 | 2119 | 4 | 1 | 6 | – | 1–2 | 3–4 | 2.60a | 5.66a | 0.21 | First meta-analysis Patients stratified: BPH alone and men with comorbid BPH and ED |
| Gacci et al [2] | 12 | 3214 | 3 | 2 | 6 | 1 | 8–12 | 2–4 | 2.85a (1.85b and c) |
5.49a (3.60b and c) |
0.01 (1.53b and c) |
First regression analysis First meta-analysis on combination of PDE5-Is and ABs |
| Yan et al [41] | 7 | 515 | 5 | – | 2 | – | 8–12 | – | 4.21a | 2.25a | 1.43a | Meta-analysis on PDE5-Is plus ABs vs PDE5-Is alone |
| Wang et al [42] | 29 | – | 9 | 13 | 2 | 5d | 8–12 | – | – | – | – | Comparison network meta-analytic study PDE5-Is alone or with AB vs: PLA, AB, 5ARI, MRA, |
| Gacci et al [43] | 8 | – | 8 | 8–12 | 2–4 | – | – | – | First review on tadalafil alone based on extrapolated data from a systematic review | |||
| Dong et al [44] | 8 | 2913 | 8 | 12 | 3–4 | 2.35a | 4.93a | +0.63* | Meta-analysis of IIEF and IPSS, including voiding and storage sub-scores Subanalysis in men with comorbid BPH + ED |
|||
| Hatzimouratidis [45] | 6 | – | 6 | 8-12 | 3–4 | – | – | – | Analysis of IPSS, voiding, and storage subscores, IPSS QoL & BPH Impact Index | |||
a Significant improvement versus placebo.
b PDE5-Is + ABs versus ABs alone.
c Significant improvement versus ABs alone.
d PDE5-Is nonspecified.
* p = 0.04 for Tadalafil 5 mg.
ABs = alpha-blockers; BPH = benign prostatic hyperplasia; diff. = difference; ED = erectile dysfunction; IIEF = International Index Of Erectile Function; IPSS = International Prostatic Symptom Score; MRAs = muscarinic receptor antagonists; O = other PDE5-Is; PDE5-Is = phosphodiesterase 5 inhibitors; PLA = placebo; P.ts = patients; Qmax = maximum flow rate at uroflowmetry; S = sildenafil; T = tadalafil;. V = vardenafil; 5ARIs = 5a-reductase inhibitors.
Moreover, we selected more clinically relevant data from current literature on the clinical use of all PDE5-Is, including all post-hoc pooled data analyses (Table 2), with a nonsystematic approach (studies published only as abstract or presented without abstract and reports from meetings and studies not published in English were not considered for this review).
Table 2 Summary of posthoc pooled-data analyses of patients treated with tadalafil 5 mg once daily versus placebo for lower urinary tract symptoms/benign prostatic enlargement
| Publication | No. of patients (n) |
Follow up (wk) |
Main findings | |
|---|---|---|---|---|
| Tadalafil | Placebo | |||
| Oelke et al., 2015 [47] | 742 | 735 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from baseline to endpoint in significantly more patients with tadalafil (69.1%) vs placebo (54.8%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from baseline to endpoint in significantly more patients with tadalafil (59.8%) vs placebo (43.7%; p < 0.001) • 59.8% (50.2%) and 79.3% (72.5%) of the responders had ≥3 (≥25%) IPSS point improvement already after wk 1 and wk 4, respectively |
| Nickel et al., 2015 [48] | 752 | 747 | 12 | Clinically-meaningful improvements of tadalafil on LUTS/BPH: • clinically-meaningful improvement (≥3 IPSS points) from randomization to endpoint in significantly more patients with tadalafil (71.1%) vs placebo (56.0%; p < 0.001) • clinically-meaningful improvement (≥25% of IPSS) from randomization to endpoint in significantly more patients with tadalafil (61.7%) vs placebo (45.5%; p < 0.001) • 38.5% (22.4%) of patients with tadalafil and 41.0% (23.3%) of patients with placebo had ≥3 (≥25%) IPSS point improvement during the placebo run-in phase |
| Oelke et al., 2014 [50] | 752 | 748 | 12 | Effects of tadalafil on nocturia (nocturnal voiding frequency): • for men with ≥1 nocturia episode at baseline, reduction with tadalafil (–0.5) was significantly greater than with placebo at study end (–0.4; LS mean change –0.2, p = 0.002) • for men with ≥2 nocturia episodes at baseline, reduction with tadalafil (–0.8) was significantly greater than with placebo at study end (–0.6; LS mean change –0.2, p = 0.003) • tadalafil (placebo) treatment resulted in reduction of ≥1 nocturia episodes in 47.5% (41.3%) of patients |
| Porst et al., 2013 [51] | 505 | 521 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • analysis only of men who were sexually active at baseline • significant improvement of total IPSS with tadalafil (–6.0) vs placebo (–3.6; p < 0.001) from baseline to study end • significant improvement of IIEF-EF with tadalafil (+6.4) vs placebo (+1.4, p < 0.001) from baseline to study end • similar IPSS improvements in men with ED vs without ED: no significant impact of ED severity on LUTS (IPSS) outcome |
| Brock et al., 2014 [52] | 752 | 744 | 12 | Effects of tadalafil on LUTS/BPH in men with vs without ED: • similar LUTS (IPSS) improvement with tadalafil vs placebo in men without ED (–5.4 vs –2.2, p = 0.0007) compared with men with ED (–5.9 vs –2.3, p < 0.0001); ED subgroup interaction not significant • bidirectional path analysis for sexually-active men (n = 1250) only: 92.5% of the total effects of tadalafil on LUTS/BPH via direct effect on LUTS and 7.5% via indirect effects by IIEF-EF domain improvement |
| Porst et al., 2013 [53] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in different patient subgroups: • improvements in IPSS and BPH-Impact Index in all patient subgroups identical (investigated patient parameters: age, symptom severity, serum testosterone concentration, prostate volume, previous α–blocker or PDE5-inhibitor use, arterial hypertension, diabetes mellitus, cardio-vascular diseases) • no baseline item was identified to predict a favorable or unfavorable treatment outcome |
| Nishizawa et al., 2015 [54] | 601 | 598 | 12 | Effects of tadalafil on LUTS/BPH in different subgroups of Asian patients: • analysis of Japanese, Korean, or Taiwanese men • significant improvements in total IPSS (tadalafil vs placebo) at wk 4 (–3.69 vs –2.47), wk 8 (–4.72 vs –3.43), and wk 12 (–5.32 vs –3.79), each p < 0.001 • additionally, significant improvements at wk 4, wk 8, and wk 12 for IPSS storage and IPSS voiding subscores as well as for IPSS-QoL • older patients (≥65 yr) showed significantly lower IPSS improvement than younger patients (<65 yr); otherwise, no significant impact of any other baseline item on LUTS improvement |
| Vlachopoulos et al., 2015 [55] | 752 | 746 | 12 | Effects of tadalafil on LUTS/BPH in patients with cardio-vascular risk factors and therapy: • subanalysis of comorbid, cardio-vascular diseases on LUTS/BPH outcome • no significant differences in efficacy of tadalafil vs placebo for various cardio-vascular diseases/comorbidities • but patients with >1 antihypertensive drugs have significantly lower IPSS improvement compared with men taking ≤1 drug • use of diuretics resulted in significantly lower IPSS improvement compared to men taking other antihypertensives or no drugs |
| Roehrborn et al., 2014 [56] | 752 (612a) |
748 (585a) |
12 | Effects of tadalafil on uroflow (maximum urinary flow rate): • in total study population, patients with tadalafil had significantly greater Qmax improvement (mean +1.1 ml/s) compared with patients with placebo (mean +0.4 ml/s; p = 0.003) • significant Qmax improvement in the total study population was mainly driven by effects in patients who voided 250–450 ml (p = 0.011) and had a baseline Qmax 10–15 ml/s (p = 0.044) |
a With valid measurement.
BPH = benign prostatic enlargement; ED = erectile dysfunction; IPSS = International Prostate Symptom Score; LUTS = lower urinary tract symptoms; PDE5 = phosphodiesterase type 5; QoL = quality of life.
Efficacy: In the first systematic review on PDE5-Is for LUTS secondary to BPH published in literature, Laydner et al [38] described a remarkable improvement of both urinary symptoms (International Prostate Symptom Score [IPSS] decrease: –2.8 to –6.3) and quality of life (IPSS quality of life [QoL] change from baseline: –0.5 to 11.7), in addition to the established effect on erectile function (International Index of Erectile Function [IIEF] increase: +6.0 to +9.17), with a negligible effect on maximum flow rate. In the first meta-analysis on PDE5-Is, Liu et al [39] reported a mean improvement in the IPSS for tadalafil, vardenafil, and sildenafil versus placebo of 5.0 versus 2.7, 5.8 versus 3.6, and 6.3 versus 1.9, respectively, with a pooled mean improvement of 5.24 versus 2.64 for placebo, in men with BPH: from the analyses of four studies including the IPSS subscores, an overall significant benefit for both irritative (–0.96%) and obstructive (–1.57%) subscore from baseline in favor of the PDE5-Is as compared with placebo has been reported. Furthermore, in men with comorbid LUTS and ED, the pooled mean improvement of IPSS score was 4.82 versus 1.94 for placebo, suggesting that PDE5-Is could be considered as the first-line treatment of patients with comorbid BPH and ED.
In the first meta-regression analysis on PDE5-Is alone or in combination with α-blockers, Gacci et al [2] evidenced that younger men, with lower body mass index and severe LUTS could be the best candidates for PDE5-Is, in terms of improvement of urinary symptoms; moreover, the combination of PDE5-Is and α-blockers significantly improved urinary symptoms (IPSS difference in mean: –1.8), erectile function (IIEF difference in mean: +3.6) and urinary flow rate (Qmax difference in mean +1.5 ml/s), when compared with the use of α-blockers alone [2]. A recent meta-analysis confirmed the significant improvement of urinary symptoms (IPSS mean difference: −4.21), sexual activity (IIEF mean difference: +2.25) and flow rate (Qmax mean difference: +1.43) with the combined therapy of PDE5-Is plus α-blockers compared with the use of PDE5-Is alone [40]. In a comparison network meta-analytic study PDE5-Is alone versus placebo allow to increase total IPSS score (mean difference [MD]: 2.1, p < 0.01), and voiding IPSS subscore (MD: 1.1, p < 0.01), while PDE5-Is with α-blockers versus placebo allow to reach a further improvement in total IPSS and both in voiding and storage subscores (MD: 5.0, p < 0.01, MD: 3.0, p < 0.01, MD: 2.2, p = 0.01), besides a significant improvement in maximum flow rate (MD: –1.9, p < 0.01) [41].
In a subset analysis on tadalafil based on data extrapolated from a systematic review on all PDE5-Is, Gacci et al [42] demonstrated a remarkable improvement of both LUTS/BPH (mean reduction of IPSS: from –1.70 to –4.10) and ED (mean improvement of IIEF: from +4.0 to +6.9) with tadalafil 5 mg once daily, evidencing a leading role for this PDE5-Is for the treatment of men with comorbid BPH and ED. In a following meta-analysis Dong et al [43] demonstrated a similar improvement of both urinary symptoms and erectile function in patients with BPH and those with comorbid BPH and ED (IPSS: –2.35 vs –2.19; IIEF: +4.93 vs +4.66, respectively); in particular, tadalafil significantly ameliorated IPSS irritative subscore (–0.86 [–1.09 to –0.64], p < 0.00001), IPSS obstructive subscore (–1.47 [–1.78 to –1.16], p < 0.00001) and IPSS-QoL (–0.35 [–0.45 to –0.24], p < 0.00001) compared with placebo. The similar improvement in total IPSS score, storage, voiding, and QoL IPSS subscores in patients with or without ED has been reported in a recent review, where the authors reported a similar improvement of BPH Impact Index in patients with or without ED (BPH Impact Index mean change from baseline:–1.6 vs –1.4, respectively) [44].
Safety: In the review from Liu et al [39], the relative risk of adverse events from tadalafil, vardenafil, and sildenafil was 2.27, 1.86, and 1.22 respectively: the overall incidence of adverse events (AEs) was 37.31% for PDE5-Is compared with 24.03% for placebo, while serious AEs were reported in 1.10% of men treated with tadalafil, 1.85% of those treated with vardenafil, and 1.05% of those treated with sildenafil.
The first meta-analysis of AEs due to PDE5-Is reported that flushing, gastro-esophageal reflux, headache, and dyspepsia had the higher risk of occurrence (odds ratio: 4.88; 2.21; 1.88; 1.85; respectively). Moreover, regarding the overall tolerability of the association between PDE5-Is and α-blockers, Gacci et al [42] described seven out of 103 AEs (6.8%) with combined therapy and five of 99 (5.1%) in men treated with α-blockers alone. Similarly, flushing (4.37%), headache (4.23%), dyspepsia (3.69%), nasopharyngitis (2.27%), and dizziness (1.69%) were the most common treatment related AEs in the comparison network meta-analytic study on PDE5-Is [41].
In a recent review on tadalafil once daily, the Authors underlined the good safety profile with a relative risk of AEs from tadalafil similar to those reported with vardenafil or sildenafil (2.27 vs 1.86 vs 1.22, respectively); overall, the occurrence of AEs reported was very similar to that reported with all PDE5-Is versus placebo: 16.0% versus 6.0% [42]. In a meta-analysis on AEs on tadalafil, Dong et al [43] reported 295 of 2338 AEs (12.6%) in men treated with tadalafil, compared with 56 of 1157 (4.8%) of those treated with placebo, with no significant difference in the incidence of serious AEs between tadalafil and placebo using the fixed-effects model. Moreover, the overall incidence of discontinuations due to AEs was 3.6% for tadalafil and 1.6% for placebo. Overall, the meta-analysis of AEs indicated a statistically significant relative risk for tadalafil versus placebo only for dyspepsia (relative risk [RR]: 11.4, p < 0.001), back pain (RR: 2.9, p < 0.001), gastro-esophageal reflux (RR: 1.9, p = 0.003), and headache (RR: 1.4, p = 0.04).
Clinically-meaningful symptom improvement: Clinically-meaningful LUTS improvement is defined as a decrease of ≥3 points or ≥25% reduction of total IPSS [45]. A post-hoc analysis of four randomized, double-blind, 12-wk studies in 1477 men investigated how many patients treated with tadalafil 5 mg once daily versus placebo achieved clinically-meaningful symptom improvement [46]. This integrated data analysis demonstrated that from baseline to study endpoint significantly more patients with tadalafil achieved an improvement of ≥3 IPSS points compared with men treated with placebo (69.1 vs 54.8%, p < 0.001) [46]. Approximatively 60% and 80% of treatment responders realized clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively. Symptom improvement ≥ 25% from baseline to study endpoint was reported by 59.8% of patients treated with tadalafil versus 43.7% of men treated with placebo (p < 0.001); of those treated with tadalafil, approximatively 50% and 73% of patients had clinically-meaningful symptom improvement after 1 wk and 4 wk, respectively.
Similar post-hoc analyses were done in 1499 patients treated with tadalafil 5 mg once daily or placebo [47]. Clinically-meaningful symptom improvement was analyzed from the start of the placebo run-in phase (in contrast to baseline [46]) to study end at wk 12. In this analysis, 81.7% of men with tadalafil and 71.2% with placebo achieved a reduction of ≥ 3 IPSS points (odds ratio 1.9, 95% CI: 1.4–2.4; p < 0.001). 38.5% of patients in the tadalafil group and 41% of patients in the placebo group reported about ≥ 3 IPSS point improvement already during the placebo-run in phase. 64.4% of men treated with tadalafil and 51.8% with placebo experienced a moderate symptom improvement (≥5.1 point reduction of the total IPSS [45]) between placebo run-in and wk 12, whereas 44.1% of men with tadalafil and 31.5% with placebo reported a marked symptom improvement (≥8.8 point reduction on the total IPSS [45]).
Nocturia: Nocturia, defined as one or more voids per night, is one of the most prevalent and bothersome single urinary symptoms in community-dwelling men or patients with LUTS/BPH and the main reason for physician consultations [48]. A post-hoc pooled analysis of 1500 patients of four randomized tadalafil trials demonstrated that 97.2% of men had ≥1 nocturnal voiding episodes at baseline (74% of men had ≥2 voiding episodes per night, ie, clinically relevant nocturia), as determined by IPSS question 7 [49]. However, the pathophysiology of nocturia was not evaluated in any of the primary tadalafil trials. Mean nocturnal voiding frequency was 2.3 ± 1.2 at baseline. Significantly more patients treated with tadalafil reported about nocturia improvement compared to patients treated with placebo (47.5% vs 41.3%, p = 0.004). Although mean nocturnal voiding frequency was only reduced by 0.5 with tadalafil and 0.4 with placebo, some patients experienced substantial improvement during tadalafil treatment (eg, ≥5 episodes at baseline to 0 or 1 episode at study end in 30.4% of patients); approximately 26% of patients with ≥2 nocturnal voids at baseline had ≤1 void at wk 12.
Tadalafil for men with or without ED: The majority of the randomized, placebo-controlled tadalafil trials included patients with ED (approximately 60–70% of trial participants). Pooled data analyses evaluated whether symptom improvement was related to baseline ED status or ED improvement during tadalafil treatment [50] and [51]. In a pooled analysis of 1026 sexually-active patients, a significant decrease of the total IPSS by 6.0 versus 3.6 points and a significant increase of IIEF-EF domain (6.4 versus 1.4) were documented for tadalafil versus placebo [50]. Patients without ED at baseline had a similar IPSS reduction compared to patient with ED; furthermore, the severity of ED did not significantly impact IPSS. The improvements of IPSS and IIEF-EF during treatments were only weakly correlated (r: –0.229). The effect of ED on LUTS reduction with tadalafil or placebo was evaluated in another integrated database containing 1496 patients [51]. IPSS and IIEF-EF significantly improved with tadalafil compared to placebo from baseline to study end at week 12 but the changes in both questionnaires were only weakly correlated (r2 0.08, p < 0.0001). Bidirectional path analyses demonstrated that IPSS improvement was largely attributed to direct (92.5%, p < 0.001) versus indirect (7.5%, p = 0.32) treatment effects via IIEF-EF improvement. Taking into account all the information from the pooled data analyses, tadalafil effects on LUTS/BPH are largely independent of ED status or improvement.
Other baseline characteristics: The impact of several patient characteristics was evaluated in 1500 pooled patients with LUTS/BPH with regard to symptom improvement and adverse events, including age (≤65 vs >65 yr), symptom severity (IPSS < 20 vs ≥ 20), testosterone concentration (<300 ng/dl vs ≥300 ng/dl), prostate-specific antigen-predicted prostate volume (<40 ml vs ≥40 ml), previous α-blockers use (yes vs no), previous PDE5 inhibitor use (yes vs no), arterial hypertension (yes vs no), diabetes mellitus (yes vs no), and cardiovascular disease (yes vs no) [52]. This pooled data analysis could not find any parameter which was significantly associated with greater symptom improvement or greater frequency of adverse events. In particular, it was shown that prostate volume determined by baseline PSA had no effect on the improvement of LUTS by tadalafil [52]. A pooled database analysis of 1199 Asian patients with LUTS/BPH showed similar results; however, patients aged ≥ 65 yr showed slightly but significantly less improvement of the total IPSS compared with younger patients (4.2 vs 5.3, p = 0.042) [53]. A recently published subanalysis investigated the impact of baseline cardiovascular diseases or cardiovascular risk factors on symptom (IPSS) improvement in 1498 patients with LUTS/BPH, including obesity (body mass index < 30 kg/m2 vs ≥ 30 kg/m2) and glycemic control (HbA1c < 6.5% vs ≥ 6.5%), number of antihypertensive medications as well as tobacco use, diabetes mellitus, insulin or oral diabetes medication use, hyperlipidemia, arterial hypertension, statin or other lipid lowering medication use, antihypertensive medication, and cardiovascular disorder [54]. No significant differences were noted for IPSS improvement except for the number of antihypertensive medication. Patients with >1 antihypertensive drugs had a significantly lower IPSS reduction compared to patients with ≤1 drug (placebo-adjusted least square mean change 1.2 vs 3.3, p = 0.02). Additionally, use of diuretics was associated with lower IPSS improvement (placebo-adjusted least square mean change –0.2 in men with diuretics vs –2.8 in men with other antihypertensive drugs or –2.3 in men without drugs).
Finally, an integrated analysis on 1371 patients, demonstrated that tadalafil 5 mg once daily for 12 wk allowed to achieve a very small, but statistically significant increase in Qmax versus the placebo (median: 1.1 ml/s vs 0.4 ml/s) [55]: significant treatment differences between placebo and tadalafil were reported only for men with baseline voided volume between 250 ml to 450 ml, while for men with volume < 250 ml or > 450 ml.
Tadalafil has been also proposed in combination with alpha blockers or 5-alpha reductase inhibitors. In a prospective randomized study on 133 men with LUTS/BPH treated with tamsulsoin plus tadalafil versus tamsulosin or tadalafil alone, combination therapy was more effective than monotherapy for ED (%IIEF change from baseline: 60.2 vs 39.3 and 46.0 respectively) with similar improvement for urinary symptoms (% IPSS change from baseline: 53.9 vs 50.9 and 33.5), with a good safety profile [56]. Moreover, in a RCT on 695 men with LUTS/BPH (610 sexually active and 450 with baseline ED) Glina et al [57] demonstrated a clinically-meaningful erectile (IIEF-EF) improvement with combination of tadalafil plus finasteride versus finasteride alone after 4 wk, 12 wk, and 26 wk of therapy in men with LUTS/BPE, regardless of the presence/absence of ED at treatment initiation.
Regarding the impact of tadalafil on sexual activity of men with ED and LUTS due to BPH tadalafil, Giuliano et al [58] demonstrated that tadalafil can preserve and improve sexual function [58]. In particular, in a RCT on Tadalafil 5 mg versus tamsulosin 0.4 mg once daily, men receiving tadalafil 5 mg once daily reported significant improvements in ejaculation, orgasm, as well as intercourse and overall satisfaction and erection: those receiving tamsulosin 0.4 mg once daily experienced a decrease in both ejaculatory/orgasmic frequency and overall satisfaction.
There is a large body of scientific evidence supporting the role of the NO-cGMP pathway including PDE5I in: (1) regulation of the tone of the smooth muscle fibers of the prostate, urethra, and bladder neck and in a lesser extent of the bladder (relaxant effect), (2) control of the arterial supply of the LUT (vasodilatory effect), and (3) modulation of the micturition reflex via the afferent bladder innervation (decrease in the afferent signaling). PDE5-Is may also downregulate inflammatory processes in LUT and exert antiproliferative effects.
Several meta-analyses demonstrated that PDE5-Is are effective in improving LUTS and erectile function compared with placebo. The combination of PDE5-Is with α-blockers induce a small, but statistically significant improvement of maximum flow rate as compared with α-blockers alone, in addition to the positive effect on micturition and sexual activity. PDE5-Is alone or in combination with α-blockers are well tolerated: flushing, gastroesophageal reflux, headache, and dyspepsia are the most common treatment-associated AEs.
Tadalafil 5 mg once daily achieves an improvement of clinically-meaningful urinary symptoms and nocturnal voiding frequency compared with placebo. Furthermore, the effects on LUTS are independent of ED severity at baseline and ED improvement.
Data on the long-term effects prostate size, reduction of disease progression, or prostate cancer prevalence related to the use of PDE5-Is are not available; long-term outcomes and cost-effectiveness analyses are needed.
Author contributions: Mauro Gacci had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gacci, Giuliano.
Acquisition of data: Gacci, Giuliano, Andersson, Maggi, Oelke.
Analysis and interpretation of data: Gacci, Andersson, Chapple, Maggi, Mirone, Oelke, Porst, Roehrborn, Stief, Giuliano.
Drafting of the manuscript: Gacci, Giuliano, Andersson, Maggi, Oelke.
Critical revision of the manuscript for important intellectual content: Chapple, Mirone, Porst, Roehrborn, Stief.
Statistical analysis: Gacci.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Giuliano.
Other: None.
Financial disclosures: Mauro Gacci certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Gacci: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Pierre Fabre; Andersson: Consultancy and Advisory Boards for Allergan, Astellas, Ferring; Chapple: Consultant, Researcher and Speaker for Allergan, Astellas, Medtronic and Recordati. Consultant and speaker for Lilly. Researcher and speaker for ONO and Pfizer. Speaker for Ranbaxy; Maggi: consultant, speaker and/or trial participant for Bayer Healthcare, GSK, Eli Lilly, Intercept, Menarini; Mirone: consultant, speaker, and/or trial participant for Eli Lilly Italia, Menarini, Zambon, Recordati, GSK, Bracco; Oelke: consultant, speaker, and/or trial participant for Bayer Healthcare, Eli Lilly and Company, and Pfizer; Porst: consultant, speaker for Eli Lilly and Compani, Menarini Group and Sanofi; CRoehrborn: has financial interests and/or other relationships with Eli Lilly and Company, and with Allergan, Afferent Pharmaceuticals, Auxiliary Medical Services, Cancer and Leukemia Group B Clinical Trial Group, GlaxoSmithKline, New England Research Institutes, NeoTract, National Institute of Diabetes and Digestive and Kidney Diseases, Protox, Southwest Oncology Group, Urologix, VA Corporate Studies, and Watson Pharmaceuticals. Giuliano: consultant for Lilly, Sanofi, Pfizer.
Funding/Support and role of the sponsor: None.