Context
There is a potential risk that testosterone replacement therapy (TRT) may exacerbate lower urinary tract symptoms (LUTS) among aging men with late-onset hypogonadism (LOH) because of testosterone’s growth-promoting effects on the prostate.
Objective
To compare the change in LUTS severity as assessed using the International Prostate Symptom Score (IPSS) between men receiving TRT versus placebo for the treatment of LOH.
Evidence acquisition
Systematic search of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library for randomized controlled trials of TRT for LOH published between January 1992 and September 2015. Studies were eligible for inclusion if they were a randomized control trial, used TRT, and assessed LUTS outcomes using the IPSS. Estimates were pooled using random effects meta-analysis. Differences by study-level characteristics were estimated using meta-regression.
Evidence synthesis
Data were extracted from 14 trials involving 2029 participants. The average age was 64.5 yr and the average follow-up was 34.4 mo. Seven studies used topical, five used injectable, and two used oral testosterone. There was no statistically significant difference in pooled changes in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% confidence interval: –0.32 to 0.55; I2 = 0%, p = 0.81], between-group difference p > 0.05). No between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone, change in testosterone, aging male symptom scale, or prostate-specific antigen levels (p > 0.05 for all comparisons).
Conclusions
In this meta-analysis of 14 clinical trials of TRT for LOH, the change in IPSS was similar among men receiving TRT versus placebo, suggesting that TRT treatment does not worsen LUTS among men with LOH.
Patient summary
In this analysis of 14 clinical trials, testosterone replacement therapy did not worsen lower urinary tract symptoms among men being treated for late-onset hypogonadism.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).
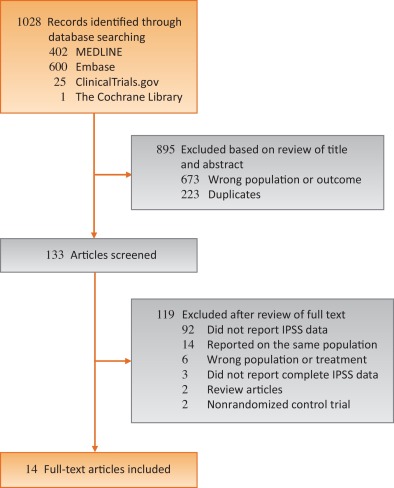
Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).
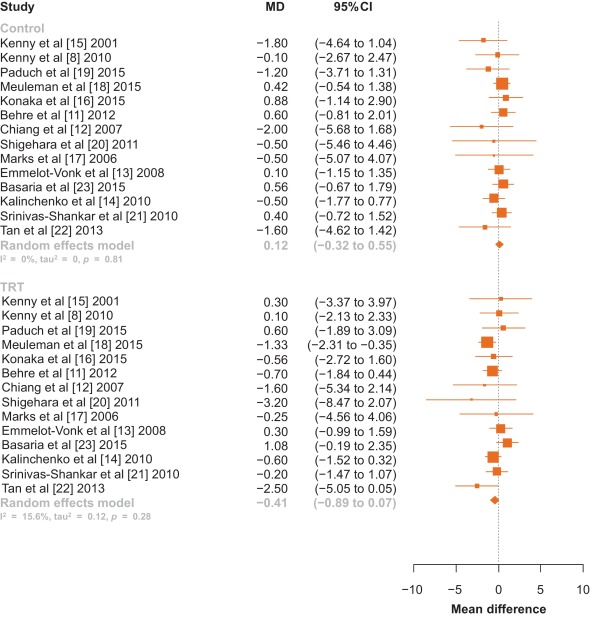
Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).
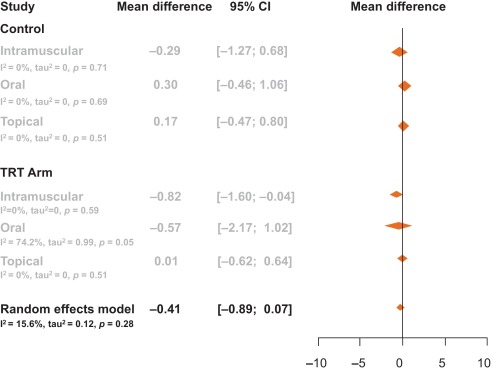
Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Longitudinal studies have demonstrated that men experience a decline in total serum testosterone beginning at 40 yr of age, and that 30% of men meet criteria for late-onset hypogonadism (LOH) by age 70 yr [1] and [2]. LOH is defined by a decrease in serum testosterone as well as symptoms such as decreased libido, depression, erectile dysfunction, and fatigue [3]. Testosterone replacement therapy (TRT), an accepted treatment for LOH, has been shown to effectively ameliorate many of its symptoms [4], [5], [6], [7], [8], and [9]. There is a theoretical risk that TRT could exacerbate lower urinary tract symptoms (LUTS). Although some studies have demonstrated that TRT does not exacerbate LUTS among men with LOH, their results have yet to be formally synthesized [8], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25]. To clarify the available evidence, we performed a systematic review and meta-analysis of randomized controlled trials to determine whether TRT affected LUTS as assessed using the International Prostate Symptom Score (IPSS).
Randomized controlled trials published between January 1992—the year the IPSS was developed [26]—and September 2015 that reported IPSS for hypogonadal men receiving TRT were identified using electronic searches of MEDLINE, Embase, ClinicalTrials.gov, and The Cochrane Library, by scanning the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [27]. A flow diagram for study selection can be seen in Figure 1. The computer-based searches combined terms related to TRT, LOH, and study design, without language restriction (full details of the search strategy are provided in Supplementary Methods 1).

Studies were included if they were a randomized controlled trials of TRT for LOH that reported on LUTS as measured by IPSS, a validated seven-question questionnaire that assesses urinary frequency, nocturia, weak stream, hesitancy, intermittent stream, incomplete emptying, and urgency on a scale of 0 to 5 [26]. The most comprehensive publication was used when there were several involving the same study population.
The following information was extracted independently by two trained investigators (T.P.K. and D.A.M.) using a standardized form: authors and publication year, participant inclusion and exclusion criteria, sample size, length of follow up, geographic locale in which the study took place, mean or median participant age, method of TRT administration, IPSS, prostate-specific antigen (PSA) levels, aging male symptoms (AMS) scale, and serum testosterone levels. All discrepancies were resolved by discussion and adjudication of a third reviewer (R.R.).
The risk of bias in the included randomized trials was assessed using the Cochrane Risk of Bias Assessment tool in the domains of randomization, sequence generation, allocation concealment, blinding, completeness of outcome data, selective outcome reporting, and other potential sources of bias [28]. Domains were independently assessed by two trained investigators (T.P.K. and D.A.M.). All discrepancies were resolved by discussion and adjudication by a third reviewer (R.R.). Risk of bias graph and summary were generated via RevMan software version 5.2. (Cochrane, Freiburg, Germany) [29].
The mean differences in IPSS measured prior to initiating and then after treatment with either TRT or placebo were calculated for each individual study. Overall differences were then calculated by pooling the study-specific estimates using random effects meta-analysis that included between-study heterogeneity [30]. Between-study heterogeneity was assessed with standard chi-square tests and the I2 statistic (ie, the percentage of variability in prevalence estimates due to heterogeneity rather than sampling error or chance) [31] and [32] and by comparing results from studies grouped according to prespecified study-level characteristics (type of testosterone, change in PSA, change in testosterone levels, and change in AMS scale) using stratified meta-analysis and meta-regression [33] and [34]. The influence of individual studies on the overall summary estimates was examined by serially excluding each study in a sensitivity analysis. Bias secondary to small study effects was investigated using funnel plot and Egger's test [35] and [36]. All analyses were performed using R Foundation for Statistical Computing 3.2.2 (The R Foundation, Vienna, Austria) [37]. Statistical tests were two-sided and used a significance threshold of p < 0.05.
Fourteen randomized controlled trials involving 2029 participants were included in this meta-analysis (Table 1). The median number of participants per study was 145 (range, 37–321), the mean age was 64.5 yr, and the mean follow up was 34.4 mo. Five studies took place in North America, four in Asia, four in Europe, and one in Australia. Two of the trials studied IPSS as a primary outcome while 12 studied it as a secondary outcome. Eight studies did not publish an IPSS score as an exclusion criterion, five studies excluded men with IPSS scores ≥ 20, and one study excluded men with IPSS scores ≥ 14. Seven studies used topical, five used injectable, and two used oral testosterone. Three studies measured serum testosterone with radioimmunoassay, three with immunochemical assays, two with chemiluminescence assays, one with gas chromatography, and one with liquid chromatography. Two studies did not specify how testosterone was measured. The majority of studies were found to be at low risk of bias (risk of bias for each individual study can be found in Supplemental Table 1).
Table 1 Selected characteristics of the 14 studies included in this systematic review
| Author | Yr | Length of follow up (wk) | Testosterone inclusion criteria | Testosterone | Sample size | Age (yr) | Change in IPSS | PSA (ng/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Dose | TRT | Control | TRT | Control | TRT | Control | TRT | Control | ||||
| Paduch et al [19] | 2015 | 16 | Total T < 300 ng/dl | Topical | 60 mg of 2% T | 30 | 35 | 52.7 | 48.4 | –1.1 | 0.5 | 0.12 | –0.04 |
| Basaria et al [23] | 2015 | 26 | Total T < 400 ng/dl or freeT <50 pg/ml | Topical | 7.5 g of 1% T | 129 | 119 | 66.9 | 68.3 | 1.02 | 0.56 | 0.69 | 0.25 |
| Meuleman et al [18] | 2015 | 28 | Free T < 0.26nmol/l | Oral | 240 mg/d T undecanoate | 69 | 71 | 58.6 | 58.4 | –1.33 | 0.42 | 0.04 | 0.03 |
| Konaka et al [16] | 2015 | 52 | Total T < 11.8nmol/l | IM | 250 mg T enanthate every 4 wk | 120 | 100 | 65.7 | 67.6 | –0.56 | 0.88 | 0.19 | 0.21 |
| Tan et al [22] | 2013 | 48 | Total T < 12nmol/l | IM | 1 g of T undecanoate at 0 wk, 6 wk, 18 wk, 30 wk, and 42 wk | 56 | 58 | 53.1 | 53.8 | –2.5 | –1.6 | 0.55 | 0.15 |
| Behre et al [11] | 2012 | 28 | Total T < 15nmol/l | Topical | 5 g of 1% T | 166 | 155 | 61.9 | 62.1 | –0.7 | 0.6 | 0.19 | –0.08 |
| Shigehara et al [20] | 2011 | 52 | Free T < 8.5 pg/ml | IM | 250 mg T enanthate every 4 wk | 23 | 23 | 72 | 68.9 | –3.2 | –0.5 | 0.322 | 0.305 |
| Kalinchenko et al [14] | 2010 | 30 | Total T < 350 ng/dl or free T < 65 pg/ml | IM | 1 g of T undecanoate at 0 wk, 6 wk, and 18 wk | 104 | 65 | 51.6 | 52.8 | –0.6 | –0.5 | 0.2 | |
| Srinivas-Shankar et al [21] | 2010 | 28 | Total T <345 ng/dl or free T < 7.2 ng/dl | Topical | 5 g of 1% T | 132 | 132 | 73.7 | 73.9 | –0.2 | 0.4 | 0.5 | |
| Kenny et al [8] | 2010 | 52 | Total T <350 ng/dL | Topical | 5 g of 1% T | 53 | 46 | 77.9 | 76.3 | 0.1 | –0.1 | –0.05 | –0.02 |
| Emmelot-Vonk et al [13] | 2008 | 28 | Total T < 13.7nmol/l | Oral | 160 mg/d T undecanoate | 113 | 110 | 67.1 | 67.4 | 0.3 | 0.1 | ||
| Chiang et al [12] | 2007 | 14 | Total T < 300 ng/dl or free T < 8.7 pg/ml | Topical | 5 g of 1% T | 20 | 17 | 47.9 | 56.1 | –1.9 | –2 | – | – |
| Marks et al [17] | 2006 | 28 | Total T < 300 ng/dl | IM | 150 mg T enanthate biweekly | 20 | 19 | 64.5 | 65.75 | –0.25 | –0.5 | 0.72 | 1.145 |
| Kenny et al [15] | 2001 | 52 | Bioavailable T < 4.44nmol/L | Topical | Two 2.5 mg transdermal T patches | 20 | 24 | 76 | 75 | 0.3 | 1.8 | 0.6 | 0.3 |
IPSS = International Prostate Symptom Score; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy; IM = intramuscular.
There was no statistically significant difference in pooled change in IPSS from baseline to follow up in men treated with TRT compared with those receiving placebo (–0.41 points [95% confidence interval: –0.89 to 0.07; I2 = 0%, p = 0.28] vs. 0.12 points [95% CI: –0.32 to 0.55; I2 = 0%, p = 0.81]; between-group difference, Q = 2.57, p = 0.11; Fig. 2). Sensitivity analysis, in which the analysis was serially repeated after the exclusion of each study, demonstrated that in the control group no individual study affected the overall prevalence estimate by more than an absolute difference of 0.08. In the TRT group, one study, Basaria et al [23] was found to affect the overall prevelnce estimate by an absolute difference of 0.21 (p = 0.01; Supplementary Table 2).

Fig. 2 Forest plot of change in International Prostate Symptom Score for men on testosterone replacement therapy verses placebo.CI = confidence interval; MD = mean difference.
Among the 14 studies, no between-group differences were noted in subanalyses that controlled for potential confounders such as type of testosterone (Fig. 3), change in PSA, change in testosterone levels, or change in AMS scale (p > 0.05 for all comparisons; Table 2).

Table 2 Meta-regression by change in prostate-specific antigen, testosterone levels, and aging male symptom score
| Control | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.02 | –0.04 | 0.00 | 3.19 | 0.07 |
| Change in PSA | –0.97 | –3.82 | 1.88 | 0.45 | 0.50 |
| Change in AMS | 0.02 | –0.35 | 0.39 | 0.01 | 0.91 |
| TRT arm | |||||
|---|---|---|---|---|---|
| Meta-regression | Slope | Lower CI | Upper CI | Q | p value |
| Change in T | –0.01 | –0.01 | 0.00 | 3.20 | 0.07 |
| Change in PSA | 1.14 | –0.76 | 3.03 | 1.38 | 0.24 |
| Change in AMS | –0.06 | –0.21 | 0.10 | 0.52 | 0.47 |
AMS = Aging Male Symptoms Scale; CI = confidence interval; PSA = prostate-specific antigen; T = testosterone; TRT = testosterone replacement therapy.
Visual inspection of the funnel plot revealed minimal asymmetry, suggesting that the pooled estimates were unlikely to be importantly biased secondary to small study effects (Supplementary Fig. 1). The Egger et al [36] regression asymmetry test supported this finding (control: z = –0.49, p = 0.62; TRT: z = –1.84, p = 0.07).
Our systematic review and meta-analysis of 14 randomized controlled trials involving 2029 men demonstrated that there was no statistically significant change in IPSS among hypogonadal men receiving TRT compared with men receiving a placebo. Neither was a clinically significant change observed as only a change in IPSS greater than 3 points as perceived by the men [38]. This negative result suggests that TRT administration does not worsen LUTS in hypogonadal men with no, mild, or moderate LUTS.
It is important to note that several of the trials excluded participants with severe LUTS, defined by an IPSS > 19. The mean IPSS among all men included in this meta-analysis had a baseline IPSS of 7.15, and there was no difference in baseline IPSS between the treatment and control groups (7.15 vs. 7.16). Changes in testosterone delivery method, PSA level, and testosterone level were not significantly associated with change in IPSS.
AMS scales were extracted from the studies to determine if an overall improvement in health caused men to notice worsened LUTS. AMS scales measure health-related quality of life and symptoms of aging men; a lower score corresponds to an improved health-related quality of life. AMS scales decreased by an average of 4.7 points in men not receiving testosterone compared with a decrease of 7.6 points in men placed on TRT. These changes, however, were not significantly associated with a change in IPSS. Therefore, men were no more aware of worsened or improved IPSS when placed on TRT when compared with men receiving a placebo.
Studies collected data on IPSS and PSA at various time intervals throughout the trial. Only one trial reported blinded results beyond 12 mo—Basaria et al [23] who reported results through 36 mo. In this study by Basaria et al [23], we selected a time point within 12 mo as a paper by Saad et al [39] suggests that the effect of testosterone on PSA and prostate volume was first observed at 3 mo after treatment initiation, and then treatment effect plateaued after 12 mo. Additionally, Saad et al [39] state that the changes in PSA and prostate volume after 12 mo is likely related to aging rather than testosterone therapy. Thus, we chose time points at or within that first 12 mo, as literature suggests that this is when TRT is primarily responsible for increases in PSA and prostate volume. In Basairia et al [23], from 6 mo to 36 mo, PSA levels increased in the control arm by 0.2 ng/dl and in the TRT arm by 0.3 ng/dl and IPSS decreased by 1.8 points in the control arm and by 0.7 points in the TRT arm. Thus, even after 36 mo of TRT, no significant or clinical changes in IPSS are observed.
The 2010 Endocrinology Clinical Society Practice Guidelines for testosterone therapy in men with androgen deficiency syndromes recommend against the use of testosterone in men with severe LUTS, defined as IPSS > 19 [40]. Only two studies to date has detailed inclusion of men with severe LUTS as many studies use IPSS > 19 as an exclusion criterion. Tan et al [22] included 10 men in the placebo arm and seven men in the treatment arm with severe IPSS (IPSS > 19), yet this study did not report changes in IPSS specifically for this group. In a retrospective study of 120 men, Pearl et al [25] found that patients with severe IPSS once started on TRT had an average decrease of IPSS of −7.42 [25]. This decrease in IPSS was found to be significant compared with patients with baseline mild and medium IPSS. This study, however, did not quantify how many patients had a baseline severe IPSS or if the patients who were prescribed medication for bothersome LUTS during the study were removed. Thus, while this study shows that TRT does not worsen IPSS, it is difficult to determine if this is due to TRT or prescribed LUTS medication. Two reviews have been published on this topic [41] and [42]. In a review of the 2010 Endocrinology Clinical Society Practice Guidelines, Seftel et al [41] called for this recommendation to be re-examined as studies seem to refute the belief that TRT exacerbates LUTS.
In this meta-analysis, only randomized controlled trials were included. One observational study was identified that had both a therapeutic group as well as a control group, but was excluded given that observational studies may be confounded by unmeasured variables [25]. No prior meta-analysis has looked primarily at changes in LUTS for men receving TRT. One earlier meta-analysis by Cui and Zhang [10] in 2013 performed a subanalysis of 346 men, looking at changes in LUTS for men on androgen replacement therapy, which found no significant difference in IPSS between men on TRT and men who were not. Our meta-analysis included a greater number of participants and studies, as well as four studies that have been published since Cui and Zhang [10]. In addition, they examined studies that used both testosterone and dihydrotestosterone therapies, while our meta-analysis focused solely on TRT.
Our study has important limitations. As mentioned, few studies examine men with severe LUTS. Additional factors such as age, prostatitis, and bladder dysfunction could also contribute to potential changes in IPSS levels. Less than 30% of studies report post-void residual volume and prostate volume, thus limiting relevant information analyzed for lower urinary tract functions. Measurements of testosterone levels were performed using various assay methods, introducing potential heterogeneity. Furthermore, to deterimine hypogonadism some studies included only men with a low serum testosterone and exhibited other hypogonadal symptoms, while other studies only required low serum testosterone levels for inclusion regardless of hypogonadal symptoms.
This meta-analysis demonstrates that in hypogonadal men with mild and moderate LUTS, no significant changes occur in IPSS when treated with TRT. Our analysis lacks hypogonadal men with severe LUTS as the current Endocrinology Clinical Society Practice Guidelines recommend against treating this population with TRT. Our meta-analysis encourages the clinical question: would hypogonadal men with severe LUTS and treated with TRT also see stable IPSS over the course of treatment as is seen in hypogonadal men with mild and moderate LUTS? Thus, we recommend that a randomized controlled trial be conducted specifically exploring the effects of testosterone on IPSS in hypogonadal men with severe LUTS.
In this meta-analysis of randomized controlled trials evaluating the effect of TRT on LUTS, the changes in IPSS were similar among men who received TRT versus those who did not. This finding suggests that hypogonadal men treated with TRT should not experience aggravation of LUTS after therapy.
Author contributions: Larry I. Lipshultz had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kohn, Mata, Ramasamy, Lipshultz.
Acquisition of data: Kohn, Mata, Ramasamy.
Analysis and interpretation of data: Kohn, Mata, Ramasamy, Lipshultz.
Drafting of the manuscript: Kohn, Mata, Ramasamy, Lipshultz.
Critical revision of the manuscript for important intellectual content: Kohn, Mata, Ramasamy, Lipshultz.
Statistical analysis: Mata, Kohn, Ramasamy.
Obtaining funding: None.
Administrative, technical, or material support: Kohn, Mata, Ramasamy, Lipshultz.
Supervision: Mata, Ramasamy, Lipshultz.
Other: None.
Financial disclosures: Larry I. Lipshultz certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.