Background
It has been shown that increased prostate size is a risk factor for lower urinary tract symptom (LUTS) progression in men who currently have LUTS presumed due to benign prostatic hyperplasia (BPH).
Objective
To determine if prostate size is a risk factor for incident LUTS in men with mild to no symptoms.
Design, setting, and participants
We conducted a post hoc analysis of the REDUCE study, which contained a substantial number of men (n = 3090) with mild to no LUTS (International Prostate Symptom Score [IPSS] <8).
Outcome measurements and statistical analysis
Our primary outcome was determination of the effect of prostate size on incident LUTS presumed due to BPH defined as two consecutive IPSS values >14, or receiving any medical (α-blockers) or surgical treatment for BPH throughout the study course. To determine the risk of developing incident LUTS, we used univariable and multivariable Cox models, as well as Kaplan-Meier curves and the log-rank test.
Results and limitations
Among men treated with placebo during the REDUCE study, those with a prostate size of 40.1–80 ml had a 67% higher risk (hazard risk 1.67, 95% confidence interval 1.23-2.26, p = 0.001) of developing incident LUTS compared to men with a prostate size 40.0 ml or smaller. There was no association between prostate size and risk of incident LUTS in men treated with 0.5 mg of dutasteride. The post hoc nature of our study design is a potential limitation.
Conclusions
Men with mild to no LUTS but increased prostate size are at higher risk of incident LUTS presumed due to BPH. This association was negated by dutasteride treatment.
Patient summary
Benign prostatic hyperplasia (BPH) is a very common problem among older men, which often manifests as lower urinary tract symptoms (LUTS), and can lead to potentially serious side effects. In our study we determined that men with mild to no current LUTS but increased prostate size are much more likely to develop LUTS presumed due to BPH in the future. This association was not seen in men treated with dutasteride, a drug approved for treatment of BPH. Our study reveals that men with a prostate size of 40.1–80 ml are potential candidates for closer follow-up.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
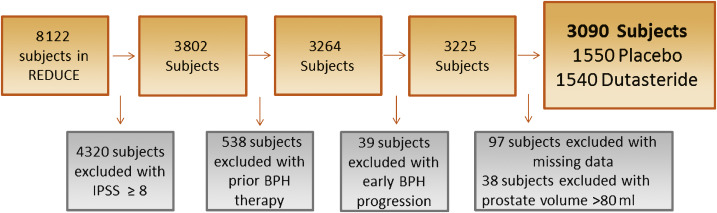
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).
Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
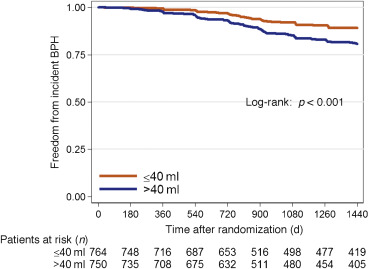
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).
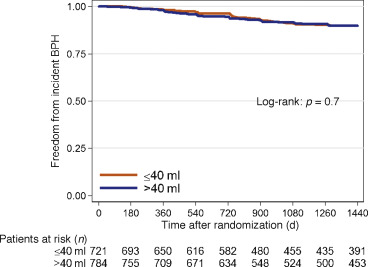
Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).
Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.
Benign prostatic hyperplasia (BPH) is a common disease in older men, with prevalence as high as 75% in men aged >70 yr, and commonly manifests as lower urinary tract symptoms (LUTS) [1]. As these symptoms can lead to depression and lower quality of life [2] and [3], as well as more serious side effects such as acute urinary retention and urosepsis [4] and [5], knowledge of factors that predict LUTS/BPH progression are paramount to prevention.
One potential risk factor for LUTS/BPH progression is an increase in prostate size [6], [7], and [8]. While there is much controversy about the correlation between prostate size and severity of symptoms [9], [10], and [11], some studies have found that increasing prostate size is significantly predictive of a higher risk of LUTS/BPH progression in men with pre-existing symptoms [6] and [7]. Notably, in a secondary analysis of the placebo arm of the Medical Therapy of Prostatic Symptoms (MTOPS) trial, Crawford et al [7] found that prostate size >30 ml predicts an increased risk of LUTS/BPH progression in men with an International Prostate Symptom Score (IPSS) ≥8 [7]. This negative effect of increased prostate size has been shown in other secondary analyses of the placebo arms of clinical trials examining the effect of various BPH treatments. Of note, subjects in these studies had pre-existing LUTS with IPSS ≥8 before enrollment [6], [7], and [8]. Among men with mild to no LUTS (IPSS <8), one study showed conflicting results with regard to prostate size predicting incident LUTS [11]. Thus, further knowledge of prostate size as a predictor of LUTS/BPH progression in men with mild to no symptoms (IPSS <8) is needed.
To address this gap in the literature, we conducted a secondary analysis of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial, which was a randomized trial of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo. A key strength of REDUCE is the prospective data on prostate size and various BPH parameters for participants who were not specifically selected with prior LUTS, including a substantial proportion of men with mild to no LUTS (IPSS <8) [12]. As enlarged prostates are associated with a higher risk of LUTS/BPH progression in symptomatic men, we hypothesized that men with enlarged prostates with mild to no LUTS are at higher risk of incident LUTS presumed due to BPH.
Our study population consisted of men from the REDUCE trial, which was a randomized, double-blind, placebo-controlled study of prostate cancer risk reduction with daily dutasteride (0.5 mg) or placebo [12]. Eligible men were aged 50–75 yr, had serum prostate-specific antigen (PSA) of ≥2.5 ng/ml (50–60 yr) or 3.0 ng/ml (60–75 yr) but ≤10 ng/ml, and one single negative prostate biopsy (6–12 cores) within 6 mo of enrollment. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, atypical small acinar proliferation, prostate volume >80 ml, previous prostate surgery, or IPSS ≥25. Men with IPSS ≥20 who were also on α-blockers or were previously on finasteride or dutasteride were excluded.
Men were required to have a negative prostate biopsy for cancer 6–12 mo before enrollment and a prostate size of ≤80 ml. Participants were followed for 4 yr, with an IPSS obtained at baseline and every 6 mo. We defined incident LUTS as the first report of medical treatment, surgery, or sustained, clinically significant BPH symptoms. Medical treatment included uroselective α-blockers (tamsulosin) and 5α-reductase inhibitors (finasteride). We defined a report of nonspecific α-blocker therapy (doxazosin, prazosin, or terazosin) as BPH if there was concomitant evidence of BPH via either self-reporting of symptoms or one report of IPSS >14 or any two reports of IPSS ≥12 at any time before the report of medication use. Surgical treatments included transurethral prostatectomy, open prostatectomy, urethral balloon dilation, and laser prostatectomy. We defined the onset of clinically significant BPH symptoms as the second report of IPSS >14 [1]. Our rationale was that IPSS of 7–18 is considered moderately symptomatic and most LUTS/BPH clinical trials use IPSS of 12 as an enrollment threshold [13]. Baseline prostate size was measured during the prestudy transrectal ultrasound (TRUS)-guided biopsy and again at the 2- and 4-yr study intervals. As our primary goal was to study men with mild to no LUTS, we excluded 4320 of the 8122 REDUCE efficacy subjects who had baseline IPSS of ≥8 (Fig. 1). We also excluded 538 participants who had previously received medical therapy (α-blockers or 5α-reductase inhibitors) for BPH. In addition, we excluded 39 subjects who met our definition of incident LUTS within 30 d of study enrollment, as this could be attributable to inaccurate reporting of the baseline IPSS. After further excluding subjects with missing data for race (n = 1), body mass index (BMI; n = 41), smoking status (n = 2), diabetes status (n = 1), prostate size (n = 48), or prostate volume >80 ml (n = 38), there were 3090 subjects remaining (Fig. 1).

Our primary aim was to determine the effect of prostate size on progression to incident LUTS among men with mild to no LUTS (IPSS <8) at baseline. Men who were diagnosed with prostate cancer during the trial were censored from the analysis at that point in time. To test our hypothesis, we used univariable and multivariable Cox proportional hazard models, as well as Kaplan-Meier plots and the log-rank test. All multivariable models were adjusted for age (continuous), race (white vs non-white), BMI (continuous), region (Europe or other vs North America), self-reported diabetes (vs none), smoking status (current, former, or never), baseline PSA (continuous), and baseline IPSS (continuous). Variables included in the multivariable analysis were not specifically chosen according to the results of the univariable analysis, and were selected if prior research demonstrated that they may moderate the relationship between prostate size and incident LUTS. We also selected demographic characteristics such as age, race, and geographic region that could moderate this effect as well [8]. We tested the linearity of prostate size in the Cox models using restricted cubic splines, and found there was no need for nonlinear terms in our models. Prostate size was examined as a continuous variable modeled per 10-ml interval and as a binomial variable (≤40 vs 40.1–80 ml). A cutoff of 40 ml was chosen based on previous research: the placebo arms of several finasteride trials demonstrated a higher risk of acute urinary retention among men with a prostate size >40 ml [14]. We also compared patient demographic characteristics between the prostate size groups (≤40 vs 40.1–80 ml) using the χ2 test for categorical variables and the rank-sum test for continuous variables.
To test for interactions between treatment and prostate size when predicting incident LUTS, an interaction term of prostate size (continuous) × treatment (dustasteride vs placebo) was created and examined in a multivariable Cox proportional hazard model adjusted for the above variables. We also tested for the interaction between baseline IPSS (continuous) and prostate size (continuous) when predicting incident LUTS in a multivariable Cox proportional hazard model adjusted for the above variables.
Statistical analysis was performed using Stata version 13.1 (Stata Corp, College Station, TX, USA). Statistical significance was two-sided at a level of p < 0.05.
Table 1 shows the demographic characteristics of the study population. Subjects had a median age of 62 yr, were predominately white (92%), lived in Europe (57%), had median PSA of 5.5 ng/ml, and median prostate size of 40.3 ml. Men with a prostate volume of 40.1–80 ml were more likely to be older, have higher BMI, be from North America, have higher baseline PSA, higher IPSS, and be a current or former smoker (all p ≤ 0.011; Table 1). During the REDUCE study, 23% of men developed prostate cancer by the 4-yr biopsy.
Table 1 Patient characteristics by prostate volume
| Variable | Total cohort | Prostate volume | p value | |
|---|---|---|---|---|
| (n = 3090) | ≤40 ml (n = 1522) |
40.1–80.0 ml (n = 1568) |
||
| Age (yr) | 62 (57–67) | 61 (57–65) | 63 (58–68) | <0.001 |
| Race | 0.11 | |||
| White | 2857 (92) | 1419 (50) | 1438 (50) | |
| Non-white | 233 (8) | 103 (44) | 130 (56) | |
| Region | 0.011 | |||
| North America | 940 (30) | 444 (47) | 496 (53) | |
| Europe | 1750 (57) | 901 (51) | 849 (49) | |
| Other | 400 (13) | 177 (44) | 223 (56) | |
| BMI (kg/m2) | 26.8 (24.9–29.3) | 26.5 (24.6–28.7) | 27.2 (25.2–29.7) | <0.001 |
| Self-reported diabetes | 242 (8) | 112 (46) | 130 (54) | 0.3 |
| Smoking status | <0.001 | |||
| Never | 1367 (44) | 675 (49) | 692 (51) | |
| Former | 1251 (41) | 577 (46) | 674 (54) | |
| Current | 472 (15) | 270 (57) | 202 (43) | |
| Baseline prostate volume (ml) | 40.3 (30.7–51.6) | NA | NA | |
| Baseline PSA (ng/ml) | 5.5 (4.2–7.1) | 5.3 (4.0–6.7) | 5.8 (4.5–7.3) | <0.001 |
| Baseline IPSS | 4 (2–6) | 4 (2–5) | 4 (3–6) | <0.001 |
| Treatment group | 0.12 | |||
| Placebo | 1550 (50) | 785 (51) | 765 (49) | |
| Dutasteride 0.5 mg | 1540 (50) | 737 (48) | 803 (52) | |
BMI = body mass index; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score.
Data are presented as median (interquartile range) for continuous variables and as n (%) for categorical variables. Comparisons between the prostate volume groups were made using the rank-sum test for continuous variables and the χ2 test for categorical variables.
Before examining the effect of prostate size on incident LUTS in the population as a whole, we first tested the interaction between treatment with dutasteride and prostate size when predicting incident LUTS. There was a significant interaction (p < 0.001), indicating that the effect of prostate size on predicting incident LUTS differed between dutasteride and placebo. Therefore, subjects were stratified by treatment group for all further analyses. We also tested the interaction between baseline IPSS and prostate size when predicting incident LUTS. However, no such interaction was found in the placebo (p = 0.3) or the treatment group (p = 0.8), so these groups were not further stratified by IPSS.
Throughout the course of the 4-yr REDUCE study, of the 1550 men in the placebo group with mild to no urinary symptoms, 193 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 124 developed incident LUTS over 4 yr, compared to 69 men with a prostate size of ≤40 ml. Of 1357 men who never developed incident LUTS, 71% had follow-up of >3 yr.
In univariable analysis, each 10-ml increase in prostate size was associated with a 17% higher risk (hazard ratio [HR] 1.17, 95% confidence interval [CI] 1.06–1.28; p = 0.001) of developing incident LUTS in the placebo group (Table 2). In multivariable analysis, each 10-ml increase in prostate size was associated with a 12% higher risk (HR 1.12, 95% CI 1.01–1.23; p = 0.031) of developing incident LUTS. A similar effect was seen when examining the effect of prostate size as 40.1–80 versus ≤40 ml in the placebo group (multivariable HR 1.67, 95% CI 1.23–2.26; p = 0.001; Fig. 2).
Table 2 Prostate size and incident lower urinary tract symptoms in the placebo group (n = 1550)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.17 (1.06–1.28) | 0.001 | 1.12 (1.01–1.23) | 0.031 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.84 (1.37–2.47) | <0.001 | 1.67 (1.23–2.26) | 0.001 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Throughout the course of the 4-yr REDUCE study, of the 1540 men in the dutasteride group with mild to no urinary symptoms, 129 developed incident LUTS. Among men with a prostate size of 40.1–80 ml, 70 developed incident LUTS over 4 yr, compared to 59 men with a prostate size of ≤40 ml. Of the 1411 men who never developed incident LUTS, 70% had follow-up of >3 yr.
In both univariable and multivariable analyses, prostate size did not predict incident LUTS regardless of whether prostate size was examined as a continuous or categorical variable (≤40 ml vs 40.1–80 ml) in the dutasteride group (all p > 0.5; Table 3 and Fig. 3).
Table 3 Prostate size and incident lower urinary tract symptoms in the dutasteride group (n = 1540)
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Baseline prostate volume per 10-ml increment | 1.04 (0.92–1.16) | 0.5 | 0.96 (0.85–1.08) | 0.5 |
| Baseline prostate volume 40.1–80.0 ml vs ≤40 ml | 1.07 (0.75–1.51) | 0.7 | 0.88 (0.62–1.27) | 0.5 |
HR = hazard ratio; CI = confidence interval.
All multivariable models were adjusted for age (continuous), race (white vs non-white), region (Europe or other vs North America), body mass index (continuous), self-reported diabetes (vs none), smoking status (current or former vs never), baseline prostate-specific antigen (continuous), and baseline International Prostate Symptom Score (continuous).

Knowledge of factors that predict incident LUTS is beneficial for prevention of future adverse outcomes such as acute urinary retention and urosepsis [4] and [5]. While it has been shown that increased prostate size is a predictor of LUTS/BPH progression in symptomatic men [7], [14], and [15], it has yet to be determined whether prostate size predicts incident LUTS in men with mild to no symptoms. To address this gap in the literature, we performed a post hoc analysis of the REDUCE trial, focusing on men with mild to no LUTS. A key strength of the REDUCE trial is the data available on baseline prostate size (as measured by TRUS) and prospectively collected IPSS scores and BPH complications [12]. Overall, among men treated with placebo with mild to no LUTS, increased prostate size was associated with a higher risk of incident LUTS. This was especially evident in men with a prostate size of 40.1–80 ml. However, the association between prostate size and incident LUTS was not observed in men treated daily with 0.5 mg of dutasteride. These findings suggest that prostate size may predict the development of incident LUTS and this effect may be negated by dutasteride use. This was evidenced by an interaction between treatment with dutasteride and prostate size when predicting incident LUTS. This means that the association between our predictor (prostate size) and outcome (incident LUTS) differed as a function of a third variable (treatment with dutasteride). In this instance, we determined that prostate size predicts incident LUTS more strongly among men treated with placebo in comparison to men treated with dutasteride.
Prior studies have shown conflicting results when correlating total prostate size to LUTS severity, with some studies showing no correlation and others showing very weak correlation [9] and [10]. In a cohort selected from the Olmsted County study of urinary symptoms and health status among men, even increasing central-zone prostate volume was only weakly correlated with IPSS and peak urinary flow rate [9].
While this lack of correlation between prostate size and LUTS severity argues against the effect of a growing prostate leading to urinary complications, several studies have highlighted the relationship between increased prostate size and LUTS/BPH progression in symptomatic individuals [5], [7], [14], and [15]. Of note, in the Olmsted County study of urinary symptoms and health status among men, those with a prostate volume >30 ml were more than twice as likely to receive treatment for BPH [15]. A secondary analysis of the placebo arm of three randomized finasteride trials demonstrated that men with a prostate size ≥40 ml were twice as likely to develop acute urinary retention at 2 yr compared to men with prostate size <40 ml [14]. In addition, a secondary analysis of the MTOPS trial, which limited enrollment to men with IPSS ≥8, demonstrated that in the placebo arm, prostate size of ≥31 ml was a significant predictor of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary incontinence, renal insufficiency, or recurrent urinary tract infection [7]. In the literature on the role of prostate size in predicting IPSS progression (one occurrence of IPSS >7 in men with IPSS ≤7 in the previous round) in men with mild to no symptoms, the Krimpen study found that prostate size was a predictor of incident IPSS progression in univariable analysis, but not multivariable analysis [11]. However, the study revealed that variables such as PSA are predictive of IPSS progression. This study differed from ours in that other parameters such as the need for BPH surgery and medical treatment were not addressed. In addition, these men had much lower PSA values (the majority had PSA <3.0 ng/ml) than in the current study, suggesting the presence of a different pathophysiology in men with higher PSA values. Furthermore, while there is a correlation between prostate size and PSA, PSA is also a marker for inflammation and is not a pure surrogate for prostate size [16].
Our findings suggest that the question of whether or not there is clinical utility in being able to predict incident LUTS in a man with mild to no symptoms must be discussed. First, and most importantly, such a prediction could potentially allow for closer follow-up in a man who perhaps would not normally be followed for LUTS/BPH because of few current symptoms. Normally, men with mild to no LUTS would not be seen in the clinic; however, the men recruited for the REDUCE trial represent a unique population. All men in this trial underwent a TRUS biopsy secondary to elevated PSA and were subsequently found to have no cancer detected on this initial biopsy. Thus, these men would be followed for prostate cancer surveillance and the size of their prostate would be known. In this specific population, men could be followed more closely for incident LUTS. In addition, these men might also be candidates for potential lifestyle modifications and could represent a potential cohort for future clinical trials.
The question of selection of men for prophylactic treatment with 5α-reductase inhibitors arises. Two studies have highlighted the benefits of treatment with 5α-reductase inhibitors in men with mild to no urinary symptoms. Parsons et al [1] examined the association between finasteride and reduction in the risk of incident LUTS using an identical definition as in the current study among men with IPSS <8, and found that treatment with finasteride led a 40% reduction in the relative risk of incident LUTS. In a post hoc analysis of the REDUCE study by Toren et al [17], men with IPSS <8 and an enlarged prostate (>40 ml) treated with dutasteride had a 15% reduction in the absolute risk of clinical BPH progression, defined as an increase in IPSS of ≥4, acute urinary retention, urinary tract infection, or surgery related to BPH. In the current study, the reduction in the absolute risk of incident LUTS in men with a prostate volume of 40.1–80 ml treated with dutasteride was 11.7%, equating to a number needed to treat (NNT) of nine. However, this benefit must be weighed against the side effects of 5α-reductase inhibitors such as loss of libido, erectile dysfunction, and gynecomastia [17]. In addition, when examining the endpoint of incident LUTS in men overall, Parsons et al [1] found a very high NNT of 58, which must be considered when making treatment decisions. On the basis of our findings and previous research, we would not recommend prophylactic treatment with 5α-reductase inhibitors to prevent incident LUTS in men with mild to no symptoms. However, these findings do suggest that prostate size is a modifiable risk for incident LUTS in men with mild to no symptoms. Future research is needed to better define LUTS prevention with an acceptable risk/benefit ratio.
A limitation of our study is that our cohort consisted of men with high PSA (median 5.5 ng/ml), so the results may not be generalizable to men with lower PSA levels. While it is plausible that the increase in LUTS is attributed to BPH, we cannot rule other factors, such as urinary tract infections and bladder dysfunction, as the cause of the observed increase in IPSS. We also could not exclude all cofounders of incident LUTS such as baseline peripheral or central nervous system abnormalities, renal dysfunction, or detrusor instability, which was not measured in this study. In addition, although definitive BPH outcomes such as acute urinary retention were recorded in the REDUCE trial, these data were not systematic and thus we could include them in our analysis. Another limitation of our study is that we used TRUS to calculate prostate size, which is not routinely performed in the clinical management of BPH. There is also evidence that the prostate shape and specifically the presence of an intravesical protrusion or middle lobe may accelerate symptom severity. We have no data to support or refute these hypotheses as the shape was not recorded [18] and [19].
Despite these limitations, our study has key strengths. First, although we used TRUS to estimate prostate size and show that larger size increases risk, we also showed that prostate size of 40.1–80 ml was significantly predictive of incident LUTS. This is relevant in that although determination of exact prostate size by digital rectal examination (DRE) is notoriously unreliable, multiple studies have shown that DRE, which is commonly performed in the clinic, is actually quite accurate in determining that a prostate is large (generally defined as >30, >40, or >50 ml) [20], [21], and [22]. Second, REDUCE contains prospective data on BPH parameters including PSA and baseline prostate size. Third, although participants were predominantly white, REDUCE was a multinational study, making it generalizable to a larger population. Fourth, although REDUCE represents a select group of men, making our study less generalizable, it does represent a population normally seen in the clinic: men who have recently had a negative biopsy after elevated PSA with minimal to no LUTS. Thus, this population represents a group of men who could be monitored for incident LUTS. However, further external validation is needed to apply the findings of our study to a general population.
In conclusion, in men with mild to no current LUTS, prostate size was associated with a higher risk of developing incident LUTS presumed due to BPH, but only among men in the placebo group. If confirmed in future studies, these findings could help in selecting men for closer follow-up who would not otherwise be followed for LUTS/BPH. The association was not observed among men on dutasteride, which suggests that dutasteride eliminates the association between prostate size and incident LUTS, essentially eliminating prostate size as a risk factor for incident LUTS.
Author contributions: Stephen J. Freedland had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal.
Acquisition of data: Simon, Howard, Moreira.
Analysis and interpretation of data: Simon, Howard, Moreira.
Drafting of the manuscript: Simon.
Critical revision of the manuscript for important intellectual content: Freedland, Simon, Howard, Roehrborn, Moreira, Vidal, Castro-Santamaria.
Statistical analysis: Simon, Howard, Moreira.
Obtaining funding: Freedland.
Administrative, technical, or material support: Freedland.
Supervision: Freedland.
Other: None.
Financial disclosures: Stephen J. Freedland certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: The REDUCE study was funded by GlaxoSmithKline.
Funding/Support and role of the sponsor: This study was supported by GlaxoSmithKline. The sponsor played a role in the design and conduct of the study and in data collection, management, analysis, and interpretation.