Context
Alpha-blockers (ABs) and 5-alpha reductase inhibitors have an established role in treating male lower urinary tract symptoms (LUTS) attributed to benign prostatic hyperplasia (BPH). Recently, newer drugs have shown promise for this indication.
Objective
To assess the comparative effectiveness and adverse effects (AEs) of newer drugs to treat LUTS attributed to BPH through a systematic review and meta-analysis.
Evidence acquisition
Ovid MEDLINE, the Cochrane Central Register of Controlled Trials, and Ovid Embase bibliographic databases (through June 2016) were hand searches for references of relevant studies. Eligible studies included randomized controlled trials published in English of newer ABs, antimuscarinics, a beta-3 adrenoceptor agonist, phosphodiesterase type-5 inhibitors, or combination therapy with one of these medications as an active comparator. Observational studies of the same agents with a duration ≥1 yr that reported AEs were also included.
Evidence synthesis
We synthesized evidence from 43 randomized controlled trials as well as five observational studies. Based on improvement of mean International Prostate Symptom Score and quality of life scores, the effectiveness of the newer ABs was not different from the older ABs (moderate strength of evidence [SOE]), but had more AEs (low SOE). Antimuscarinics/AB combination therapy had similar outcomes as AB monotherapy (all moderate SOE), but often had more AEs. Phosphodiesterase type-5 inhibitors alone or in combination with ABs had similar or inferior outcomes than ABs alone. Evidence was insufficient for the beta-3 adrenoceptor agonist. For all newer agents, the evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs.
Conclusions
None of the drugs or drug combinations newly used to treat LUTS attributed to BPH showed outcomes superior to traditional AB treatment. Given the lack of superior outcomes, the studies’ short time-horizon, and less assurance of their safety, their current value in treating LUTS attributable to BPH appears low.
Patient summary
In this paper, we reviewed the evidence of newer drugs to treat men with urinary problems attributable to an enlarged prostate. We found none of the new drugs to be better but there was more concern about side effects.
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.
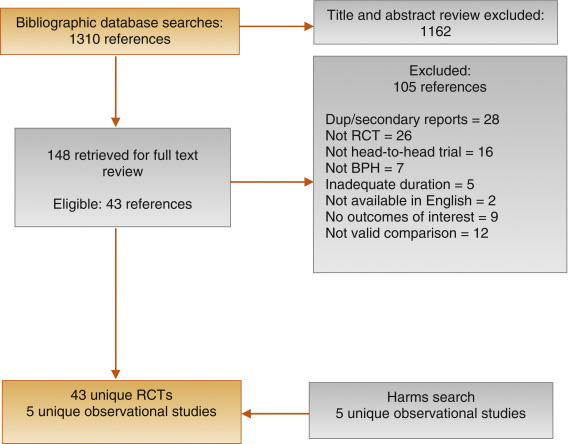
Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).
Alpha-blockers (ABs) and 5-alpha reductase inhibitors (5-ARIs) have an established role in treating lower urinary tract syptoms (LUTS) attributed to benign prostatic hyperplasia (BPH) 1 2 3 4 5 6 . Recently, a new AB and drugs in other classes approved for different indications have shown promise in this setting. The purpose of our review was to determine the comparative effectiveness and safety of medications newly used in the last 10 yr for LUTS attributed to BPH, both as single agents and in combination.
We developed an a priori written protocol, together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment (available at the Agency for Healthcare Research and Quality [AHRQ] website http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?productid=2067&pageaction=displayproduct ).
Based on our Population, Interventions, Comparisons, Outcomes, Timing, and Setting criteria (Supplementary data) we included randomized controlled trials (RCTs) that tested comparative effectiveness of treatments involving newer drugs in men aged ≥45 yr with LUTS attributed to BPH. We defined these newer drugs as those that were Food and Drug Administration (FDA) approved for BPH since 2008 or which, though not FDA approved for BPH, have been studied for the treatment of BPH since 2008 and were selected through a formal process of stakeholder involvement (Supplementary data). Comparators included medications FDA approved for BPH before 2008. Included RCTs were at least 1 mo in duration with no minimum sample size. We additionally searched for large ( n ≥ 100 patients), longer-term (≥ 1 yr duration) observational studies to assess long-term or rare treatment associated harms only. We limited inclusion to English language articles.
The primary predefined outcomes of interest were changes reflecting clinically important differences (Supplementary data) in validated measures to assess LUTS (International Prostate Symptom Score [I-PSS]: score ranges 0–35 with higher scores indicating more severe symptoms; or American Urological Association Symptom Index scores), prostate-related bother or quality of life (QoL; I-PSS QoL question; BPH/LUTS impact scale), as well as rates of disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention [AUR]). We also assessed common and serious medication adverse effects (AEs).
We searched Ovid Medline, Ovid Embase, and the Cochrane Central Register of Controlled Trials with filters for study design (Supplementary data), to identify relevant RCTs published through June 20, 2016. We also searched for relevant systematic reviews and other key references. Lastly, we searched the Clinical Trials ( www.clinicaltrials.gov ) and the FDA ( www.fda.gov/Drugs ) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using AHRQ guidance by one investigator and reviewed by a second [7] .
We assessed clinical and methodological heterogeneity and variation in effect size to determine the appropriateness of pooling data [8] . When three or more trials reported similar comparisons and outcomes, data were pooled using a Hartung, Knapp, Sidik, and Jonkman method [9] random effects model for proportion of I-PSS responders or mean changes in I-PSS scores in Stata (StataCorp., College Station, TX, USA). We pooled other outcomes in RevMan (RevMan, Spartanburg, SC, USA) [10] and converted DerSimonian-Laird random effects confidence intervals to Hartung, Knapp, Sidik, and Jonkman confidence intervals using an excel spreadsheet provided in Inthout et al [9] . We assessed between study variance with Tau 2 and measured the magnitude of heterogeneity with the I 2 statistic. If substantial heterogeneity was present (ie, I 2 ≥ 70%), we stratified the results to assess treatment effects based on patient or study characteristics and/or explored sensitivity analyses [8 11] . We pooled across different ABs unless there were at least three trials for a given agent. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical significance (Supplementary data).
For the body of evidence from RCTs, we rated our confidence in the estimates of effect for the primary outcomes as overall strength of evidence (SOE) as high, moderate, low, or insufficient (Supplementary data) [12] . For observational studies, we did not formally assess SOE, but provided descriptive information in narrative form.
Our literature search identified 1139 references, of which 124 were selected for full-text review ( Fig. 1 ). This process mapped to 43 unique RCTs. In addition, we identified five longer duration (≥ 1 yr) observational studies reporting AEs.

Fig. 1
Figure 1: Literature flow diagram. The results are presented separately for each of four drug classes (new alpha-blockers, anticholinergics, beta-3 agonists, and phosphodiesterase type-5 inhibitor), and specific drugs are listed within each class. The outcomes addressed by the three key questions are discussed within each drug-specific section.
BPH = benign prostatic hyperplasia; RCT = randomized controlled trial.
Ten trials randomized men with LUTS attributed to BPH ( n = 1799) to silodosin 8 mg daily versus tamsulosin 0.2–0.4 mg daily 13 14 15 16 17 18 19 20 21 22 . Table 1 provides baseline characteristics. Overall, the RoB was low in two trials [13 18] , moderate in six trials 14 15 16 20 21 22 , and high in two trials [17 19] .
| Study, [reference]/location | Intervention (daily dosage) | Control (daily dosage) | No. randomized | Duration (wk) | Mean Age (yr) | Mean I-PSS |
|---|---|---|---|---|---|---|
| Alpha-blockers, silodosin versus tamsulosin | ||||||
| Takeshita (2016) [21] /Japan | Silodosin 4 mg | Tamsulosin 0.2 mg | 34 | 4 wks a | 70 | 16 |
| Manjunatha (2016) [22] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 60 | 12 wks | 64 | 19 |
| Pande (2014) [13] /India | Silodosin 8 mg | Tamsulosin 0.4 mg | 61 | 12 wk | 62 | 18 |
| Chapple (2011) [18] /Europe | Silodosin 8 mg | Tamsulosin 0.4 mg | 765 | 12 wk | 66 | 19 |
| Yokoyama (2012) [14] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 46 | 12 wk a | 69 | 20 |
| Watanabe (2011) [17] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 102 | 4 wk a | 70 | 17 |
| Yokoyama (2011) [16] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 90 | 12 wk | 71 | 18 |
| Yu (2011) [15] /Taiwan | Silodosin 8 mg | Tamsulosin 0.2 mg | 209 | 12 wk | 66 | 20 |
| Miyakita (2010) [19] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 65 | 4 wk a | 69 | 17 |
| Kawabe (2006) [20] /Japan | Silodosin 8 mg | Tamsulosin 0.2 mg | 367 | 12 wk | 66 | 17 |
| Totals and means | 1799 | 67 | 18 | |||
| Anticholinergics, fesoterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Konstantinidis (2013) [26] /Greece | Fesoterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 47 | 4 wk | 64 | 16 |
| Kaplan (2011) [25] /Multinational | Fesoterodine 4 mg +/vs | Various | 943 | 12 wk | 66 | 19 |
| Totals and means | 990 | 66 | 19 | |||
| Anticholinergics, solifenacin plus alpha-blocker versus alpha-blocker tamsulosin monotherapy | ||||||
| Van Kerrebroeck (SATURN) (2013) [33] /Europe | Solifenacin 3–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 715 | 12 wk | 65 | 18 |
| Van Kerrebroeck (NEPTUNE) [32] (2013)/Europe | Solifenacin 6–9 mg +/vs | Tamsulosin 0.4 mg OCAS | 993 | 12 wk | 65 | 19 |
| Kaplan (2009) [29] /USA | Solifenacin 5 mg +/vs | Tamsulosin 0.4 mg | 398 | 12 wk | 65 | 17 |
| Ko (2014) [28] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 187 | 12 wk | 61 | 19 |
| Lee (2014) [30] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 156 | 12 wk | 61 | 18 |
| Seo (2011) [31] /Korea | Solifenacin 5 mg +/vs | Tamsulosin 0.2 mg | 60 | 12 wk | 58 | 18 |
| Yamaguchi (2011) [34] /Japan | Solifenacin 2.5–5 mg +/vs | Tamsulosin 0.2 mg | 638 | 12 wk | 70 | 14 |
| Totals and means | 3147 | 66 | 17 | |||
| Anticholinergics, tolterodine plus alpha-blocker versus alpha-blocker monotherapy | ||||||
| Memon (2014) [38] /India | Tolterodine 4 mg +/vs. | Alfuzosin 10 mg | 70 | 12 wk | 62 | 24 |
| Lee (2011) [37] /Korea | Tolterodine 4 mg ER +/vs | Doxazosin 4 mg GITS | 176 | 12 wk | 61 | 21 |
| Chapple (2009) [35] /Multinational b | Tolterodine 4 mg ER +/vs | Various | 652 | 12 wk | 65 | 19 |
| Kaplan (2006) [36] /USA | Tolterodine 4 mg ER +/vs | Tamsulosin 0.4 mg | 440 | 12 wk | 61 | 20 |
| Totals and means | 1338 | 63 | 20 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker tamsulosin monotherapy | ||||||
| Oelke (2012) [43] /Multinational | Tadalafil 5 mg | Tamsulosin 0.4 mg | 339 | 12 wk | 64 | 17 |
| Yokoyama (2013) [42] /Asia | Tadalafil 2.5–5 mg | Tamsulosin 0.2 mg | 458 | 12 wk | 63 | 17 |
| Kim (2011) [44] /Korea | Tadalafil 5 mg | Tamsulosin 0.2 mg | 100 | 12 wk | 61 | 17 |
| Singh (2014) [41] /India | Tadalafil 10 mg | Tamsulosin 0.4 mg | 89 | 12 wk | 61 | 21 |
| Totals and means | 986 | 63 | 17 | |||
| Phosphodiesterase type 5 inhibitors, tadalafil versus alpha-blocker monotherapy | ||||||
| Kumar (2014) [45] /India | Tadalafil 10 mg | Alfuzosin 10 mg | 50 | 12 wk | 62 | 18 |
| Liguori (2009) [46] /Italy | Tadalafil 20 mg (taken alternated d) | Alfuzosin 10 mg ER | 43 | 12 wk | 61 | 15 |
| Totals and means | 93 | 62 | 16 | |||
a Crossover study, first phase presented only.
b Includes several continents.
ER = extended release; GITS = gastrointestinal therapeutic system; I-PSS = International Prostate Symptom Score; OCAS = oral controlled absorption system; vs = versus.Table 1: Baseline characteristics of eligible comparative effectiveness trials
Three trials conducted responder analyses (defined as > 25% reduction in I-PSS score; Table 2 ) [16 18 20] . Response to treatment was not significantly different between silodosin and tamsulosin (risk ratio: 1.07, 95% confidence interval [CI]: 0.91–1.26; moderate SOE). Silodosin and tamsulosin (all dose levels) were not significantly different in improving mean I-PSS scores (weighted mean difference [WMD]: –0.52, 95% CI: –1.58 to 0.54; moderate SOE). Results were similar in analyses restricted to trials using tamsulosin 0.4 mg.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, based on ≥25% reduction in total I-PSS score from baseline | 3 (1283) | 72 (456/632) | 68 (440/651) | Similar between groups: RR 1.07 (0.91–1.26) | Moderate a |
| I-PSS score, mean change from baseline | 8 (1598) | –8.2 points | –7.5 points | Similar between groups: WMD –0.52 (–1.58 to 0.54) | Moderate a |
| I-PSS QoL, mean change from baseline | 6 (788) | –1.7 points | –1.4 points | Similar between groups: WMD –0.20 (–0.72 to 0.32) | Moderate a |
| Overall withdrawals | 4 (1125) | 9 (53/563) | 9 (49/562) | Similar between groups: RR 1.05 (0.73–1.5) | Low a , b |
| Withdrawals due to adverse effects | 3 (1222) | 5 (30/601) | 3 (16/621) | Greater with silodosin: RR 1.96 (1.04–3.71) | Moderate a |
| Participants with ≥1 adverse effect | 3 (1338) | 52 (342/659) | 46 (314/679) | RR 1.11 (0.95–1.29) | Insufficient |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 2Evidence overview of silodosin 8 mg versus tamsulosin 0.2–0.4 mg
Overall withdrawals (for any reason) and rates of participants with ≥1 AEs were not significantly different between treatments (low and moderate SOE, respectively). Withdrawals due to AEs were more frequent for silodosin versus tamsulosin (risk ratio: 1.96, 95% CI: 1.04–3.71; moderate SOE). The most common AE was abnormal ejaculation, reported in 16% of patients on silodosin versus 2% of patients on tamsulosin.
Two 146 12-wk trials ( n = 161) compared darifenacin/AB combination 147 therapy with AB monotherapy in men with LUTS and overactive bladder (OAB) symptoms attributed to BPH [23 24] . Participants with a baseline postvoid residual of >150 ml were excluded. RoB was low in one trial [24] and moderate in the other [23] . SOE was judged insufficient for all outcomes.
Two trials ( n = 990) compared fesoterodine/AB combination therapy with AB monotherapy ( Table 1 ) in men with LUTS and OAB symptoms [25 26] . Overall RoB was moderate for one trial [25] and high for the other [26] . Improvement in mean I-PSS scores was similar with fesoterodine/AB combination and AB monotherapy (low SOE; Table 3 ). The mean difference in the large moderate RoB trial was 0.0 (95% CI: –0.83 to 0.83) and –1.70 (95% CI: –5.85 to 2.46) for the small high RoB trial. Overall withdrawals, withdrawals due to AEs, and the number of participants with ≥1 AE were more frequent in the fesoterodine arm; SOE was judged as low for all three outcomes.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 2 (990) |
Range –2.4 to –4.4 |
Range –0.7 to –4.4 |
Studies not pooled. Both were similar to control | Moderate a |
| I-PSS QoL, mean change from baseline | Not reported | Insufficient | |||
| Overall withdrawals | 1 (947) | 15 (73/474) | 10 (49/473) | Greater with fesoterodine: RR 1.49 (1.06–2.09) | Low a , b |
| Withdrawals due to adverse effects | 1 (947) | 10 (46/474) | 4 (20/473) | Greater with fesoterodine: RR 2.30 (1.38–3.82) | Low a , b |
| Participants with ≥1 adverse effect | 1 (947) | 49 (230/474) | 33 (157/473) | Greater with fesoterodine: RR 1.46 (1.25–1.71) | Low a , b |
a Risk of bias (moderate).
b Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 3Evidence overview of fesoterodine 4 mg plus various alpha-blockers versus various alpha-blockers monotherapy
One 12-wk trial ( n = 420) trial compared oxybutynin 10 mg tablets plus tamsulosin 0.4 mg with tamsulosin 0.4 mg alone [27] . Individuals with a baseline postvoid residual of >200 ml were excluded. RoB was moderate. SOE was judged insufficient for all outcomes.
Seven 12-wk trials 28 29 30 31 32 33 34 randomized men with LUTS and OAB symptoms ( n = 3147) to solifenacin plus tamsulosin versus tamsulosin monotherapy ( Table 1 ). Five trials examined solifenacin 5 mg 28 29 30 31 34] and two examined solifenacin 6 mg [32 33] . The overall RoB was moderate.
Combination therapy was similar to AB monotherapy in improving LUTS (moderate SOE; Table 4 ). Improvement in mean I-PSS score from baseline was similar with solifenacin 5 mg or 6 mg plus tamsulosin 0.2 mg or 0.4 mg versus tamsulosin alone (WMD: –0.29, 95% CI: –0.88 to 0.30; moderate SOE). Combination therapy lowered I-PSS QoL score more than tamsulosin, but the difference between groups was not clinically significant based on predetermined thresholds (moderate SOE). Evidence from four trials using solifenacin 3–9 mg [29 32 33 34 reported no statistical difference between treatment groups in rates of AUR, but evidence was judged insufficient to draw conclusions due to imprecision.
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 6 (1948) | –5.8 points | –5.4 points | Similar between groups: WMD –0.29 (–0.88–0.30) | Moderate a |
| I-PSS QoL, mean change from baseline | 4 (1225) | –1.2 points | –0.9 points | Similar between groups: WMD –0.18 (–0.39 to –0.03) | Moderate a |
| Overall withdrawals | 7 (3147) | 10 (203/2028) | 11 (121/1119) | Similar between groups: RR 1.02 (0.74–1.41) | Low a,b |
| Withdrawals due to adverse effects | 5 (2900) | 4 (71/1904) | 3 (30/996) | Similar between groups: RR 1.27 (0.81–2.0) | Low a,b |
| Participants with ≥1 adverse effect | 5 (2918) | 33 (623/1913) | 29 (280/1005) | Greater with combined therapy: RR 1.21 (1.09–1.35) | Moderate a |
a Risk of bias (moderate).
b Imprecision.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 4Evidence overview of combined solifenacin 5–6 mg plus tamsulosin 0.2–0.4 mg versus tamsulosin 0.2–0.4 mg monotherapy
Withdrawal for any reason or due to AEs was similar with both treatments (low SOE). More participants reported one or more AEs with combination treatment than monotherapy (moderate SOE).
Four trials randomized men with LUTS and OAB symptoms ( n = 1338) to a combination of tolterodine 4 mg plus AB versus AB monotherapy with tamsulosin, doxazosin, or alfuzosin ( Table 5 ) 35 36 37 38 . Overall RoB for three trials was low 35 36 37 and one trial had a high RoB [38] .
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders, defined as a 3-point improvement in I-PSS score from baseline | 1 (70) | 77 (27/35) | 29 (10/35) | RR 2.7 (1.55–4.70) | Insufficient a , c |
| I-PSS score, mean change from baseline | 4 (1249) | –5.9 points | –5.6 points | Similar between groups: WMD –0.19 (–1.08 to 0.69) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (1182) | –1.3 points | –1.1 points | Similar between groups: WMD –0.34 (–1.14 to 0.46) | Low b , c |
| Overall withdrawals | 3 (1268) | 16 (101/639) | 14 (88/629) | Similar between groups: RR 1.11 (0.53–2.34) | Low b |
| Withdrawals due to adverse effects | 3 (1268) | 6 (36/639) | 3 (16/629) | RR 2.17 (0.93–5.06) | Insufficient b |
| Participants with ≥1 adverse effect | 1 (652) | 35 (114/329) | 28 (89/323) | RR 1.26 (1.00–1.58) | Insufficient b , c |
a Risk of bias (moderate or high).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 5Evidence overview of combined tolterodine 4 mg plus various alpha-blockers versus various alpha-blocker monotherapy
Responder analysis was provided in only the high RoB trial [38] with a response defined as a 3-point improvement in I-PSS score from baseline ( Table 3 ). The proportion of responders was greater in the combination group than the AB monotherapy group (77% vs 29%), but the SOE for the comparison was judged as insufficient for this outcome due to a high RoB and unknown consistency. Mean changes in I-PSS scores were similar between treatment groups (WMD: –0.19, 95% CI: –1.08 to 0.68; moderate SOE). The mean change in I-PSS QoL was also not different between treatment groups (WMD: –0.34, 95% CI: –1.14 to 0.46; low SOE) 35 36 37 .
There were six incidences of AUR in the combination group versus two in the monotherapy group, which was judged as insufficient evidence 35 36 37 . Withdrawal for any reason (low SOE), withdrawal due to adverse effects (low SOE), and rates of ≥1 AEs (insufficient SOE) were not different between groups [35] .
One 12-wk trial ( n = 58) compared trospium 45 mg daily doses with AB to AB monotherapy in men with LUTS and OAB symptoms attributed to BPH [39] . Individuals with a baseline postvoid residual of >100 ml were excluded. RoB was moderate.
Evidence was insufficient to assess efficacy for any outcome. One or more AE were reported in nine (35%) trospium participants versus five (23%) placebo patients.
We found one 8-wk trial [40] ( n = 94), which compared 50 mg of mirabegron combined with 0.2 mg tamsulosin versus tamsulosin monotherapy in males with LUTS and OAB symptoms attributed to BPH. Patients with postvoid residual urine volume >100 ml were excluded. The study was judged to be at high RoB. The SOE was judged insufficient for all predetermined patient-important outcomes. We found no comparative trials combining mirabegron with other ABs for this indication.
Four 3-mo trials compared tadalafil 2.5 mg, 5 mg, or 10 mg daily with tamsulosin 0.2 mg or 0.4 mg daily ( Table 1 ) 41 42 43 44 . Most participants had a history of erectile dysfunction (ED) [41 43 44] . The most frequently investigated dose level of tadalafil was 5 mg; one trial included a 2.5-mg dose level [42] and one trial evaluated 10 mg [41] . Overall RoB ranged from low to high for the four trials.
Tadalafil 5 mg and tamsulosin were similar in improving mean I-PSS scores (WMD: –0.07, 95% CI: –2.12 to 2.23; moderate SOE) and I-PSS QoL (WMD: –0.01, 95% CI: –0.75 to 0.73; low SOE). Evidence was insufficient for the outcomes of study withdrawal for any reason and the proportion of participants reporting at least one AE, but withdrawal due to AEs was higher with tadalafil (3% vs 1%; moderate SOE; Table 6 ).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
| I-PSS score, mean change from baseline | 3 (742) | –5.6 points | –5.9 points | Similar between groups: WMD –0.07 (–2.12 to 2.23) | Moderate a |
| I-PSS QoL, mean change from baseline | 3 (742) | –1.1 points | –1.1 points | Similar between groups: WMD –0.01 (–0.75 to 0.73) | Low a , c |
| Overall withdrawals | 3 (742) | 10 (36/373) | 8 (28/369) | Similar between groups: RR 1.35 (0.30–6.05) | Low a , b |
| Withdrawals due to adverse effects | 3 (742) | 3 (11/373) | 1 (4/369) | Greater with tadalafil: RR 2.68 (1.09–6.60) | Moderate a |
| Participants with ≥1 adverse effect | 3 (742) | 25 (94/373) | 24 (90/369) | Similar between groups: RR 0.99 (0.38–2.56) | Low a , b |
a Risk of bias (moderate).
b Imprecision.
c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 6Evidence overview of tadalafil 5 mg versus tamsulosin 0.2–0.4 mg monotherapy
Two 3-mo trials ( n = 93) compared tadalafil with alfuzosin 10 mg daily ( Table 1 ) [45 46] . Kumar et al [45] compared tadalafil 10 mg daily with alfuzosin 10 mg daily. Liguori et al [46] compared tadalafil 20 mg taken on alternate days with alfuzosin 10 mg daily. All participants had a history of ED. Both trials were open label with a high overall RoB.
Alfuzosin 10 mg improved mean I-PSS scores more than tadalafil 10 mg or 20 mg (low SOE; Table 7 ). Mean reductions in I-PSS scores were 4.1 and 7.2 points with tadalafil and alfuzosin, respectively, favoring alfuzosin. I-PSS QoL also improved more with alfuzosin than tadalafil (low SOE). Study withdrawal for any reason and withdrawal due to an AE were no different with tadalafil or alfuzosin (insufficient SOE).
| Outcome | No. of trials (evaluated) | Intervention, % ( n / N ) or mean | Control, % ( n / N ) or mean | Results and magnitude of effect (95% CI) | Strength of evidence |
|---|---|---|---|---|---|
| Responders | Not reported | Insufficient | |||
I-PSS score, mean change from baseline |
2 (87) |
Range –1.3 to –6.3 |
Range –5.2 to –9.5 |
Studies not pooled. Both favored alfuzosin. | Low a , b |
I-PSS QoL, mean change from baseline |
2 (87) |
Range –1.0 to –2.4 |
Range –1.3 to –3.2 |
Studies not pooled. Both favored alfuzosin | Low a , b |
Overall withdrawals |
2 (87) | Range (%) 0–10 |
Range (%) 0–18 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Withdrawals due to adverse effects | 2 (87) | Range (%) 0–5 |
Range (%) 0–14 |
Studies not pooled. No events in one trial and very wide CIs in other trial. | Insufficient |
| Participants with ≥1 adverse effect | Not reported | Insufficient |
a Risk of bias (moderate).
b Imprecision. c Unknown consistency or inconsistency.
CI = confidence intervals; I-PSS = International Prostate Symptom Score; QoL = quality of life; RR = risk ratio; WMD = weighted mean difference.Table 7Evidence overview of tadalafil 10–20 mg versus alfuzosin 10 mg monotherapy
Three trials ( n = 181) compared sildenafil versus an AB 47 48 49 . One compared sildenafil 25 mg daily with alfuzosin 10 mg daily over 12 wk [48] and one compared sildenafil 25 mg taken 4 d/wk with tamsulosin 0.4 mg daily over 8 wk [49] . Abolyosr et al [47] compared sildenafil 50 mg to a low dose of doxazosin 2 mg over 16 wk; frequency of administration was not reported. All participants had a history of ED. All trials were open label and the overall RoB was high.
Mean change in I-PSS scores was –2.2 with sildenafil and –3.2 with alfuzosin or doxazosin (insufficient SOE) [47 48] . Mean change in I-PSS scores with sildenafil 25 mg was 4.0 points versus 5.4 points for tamsulosin 0.4 mg (insufficient SOE) [49] .
Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 224) compared tadalafil combined with an AB versus AB monotherapy [41 45 46 50] . Two 3-mo trials compared tadalafil 10 mg daily [45] or 20 mg on alternate days [46] combined with alfuzosin 10 mg to alfuzosin 10 mg monotherapy. Two trials evaluated tadalafil combined with tamsulosin 0.4 mg versus tamsulosin 0.4 mg monotherapy: a 1-mo trial evaluated tadalafil 5 mg daily [50] and a 4-mo trial evaluated tadalafil 10 mg daily [41] . Nearly all participants had a history of ED [41 45 46] . All trials were open label except Regadas et al [50] and the overall RoB therefore ranged from moderate to high.
Results with combination therapy were similar to AB monotherapy (tadalafil 5–20 mg combined with AB was no different than AB monotherapy in improving mean I-PSS scores from baseline [WMD: –2.0, 95% CI: –4.03 to –0.00; insufficient SOE]). Mean reductions in I-PSS scores were similar; 10.4 and 8.6 with combination and monotherapy. Improvement in mean I-PSS QoL scores was also not significantly different between combination treatment and monotherapy; however, only open label (high RoB) trials reported this outcome (low SOE) [41 45 46] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
Four trials ( n = 281) compared sildenafil combined with an AB versus AB monotherapy 47 48 49 51] . Two 3-mo trials evaluated sildenafil combined with alfuzosin 10 mg versus alfuzosin monotherapy; one used daily sildenafil 25 mg [48] and the other used sildenafil 50 mg (dosing frequency not reported) [51] . One 4-mo trial evaluated sildenafil 50 mg combined with doxazosin 2 mg versus doxazosin alone, but dosing frequency was not reported [47] . An 8-wk trial evaluated sildenafil 25 mg taken 4 d/wk combined with tamsulosin 0.4 mg daily compared with tamsulosin monotherapy [49] . Three trials enrolled men with a history of ED 47 48 49 . All trials were open label or otherwise inadequately blinded and enrolled patients after they failed to respond to AB monotherapy. Overall RoB was mostly high.
Mean reductions in I-PSS scores were 5.4 with combination and 3.9 with monotherapy, with both treatments exceeding MDD. However, the SOE to assess the comparative effectiveness was insufficient due to the study limitations and imprecision in measurement [47 48 51] . In the 8-wk trial without data sufficient for pooling, improvement in mean I-PSS scores was similar with combination or monotherapy (–6.4 vs –5.4) [49] . Evidence was insufficient for overall withdrawals and withdrawals due to AEs.
One double-blinded trial ( n = 60) compared vardenafil 10 mg daily combined with tamsulosin 0.4 mg to tamsulosin monotherapy over 12 wk [52] . The overall RoB was moderate. Mean reductions in I-PSS scores were 5.8 with combination treatment and 3.7 with monotherapy, both achieving MDD (insufficient SOE).
One withdrawal was reported with tamsulosin. No participant withdrew due to AEs. Persistent AEs were reported in three participants with combination therapy (headache with flushing, headache with stomach pain, and stomach pain) and two with tamsulosin (headache and flushing). No serious AEs were reported.
The evidence from a single trial of tadalafil 5-ARI combination versus 5-ARI monotherapy [53] as well as that from a single trial comparing tadalafil versus 5-ARI/AB combination [54] was insufficient for both comparative effectiveness and safety.
We identified two observational studies reporting longer-term AEs related to silodosin treatment [55 56] . In a 40-wk open label extension (cumulative treatment duration of 52 wk) of a previous RCT, 435 patients completed the extension in which a total of 431 experienced 924 AEs [55] . Twenty-nine patients (4.4%) experienced serious AEs including two deaths; none of the serious AEs were considered drug related, although no criteria were reported. The second study reviewed FDA data for AEs associated with ABs and found the evidence of silodosin insuffient to compare with other ABs [56] .
We identified one study examining long-term AEs associated with solifenacin/AB combination therapy, reporting an open extension from a previous RCT for a subset of patients with inclusion criteria that included a postvoid residual ≤150 ml [57] . Forty-seven percent of participants who continued solifenacin/AB combination treatment reported treatment-emergent AEs, most commonly dry mouth, constipation, and dyspepsia. In addition, 86 serious AEs occurred in 64 patients and included three deaths, six cases of AUR (0.7%), and three cases of intervertebral disc protrusion.
We found two longer-term observational studies on tadalafil [58 59] . In a 42-wk open label extension of a previous trial, 59% of 394 participants reported at least one AE and 9% withdrew due to an AE. Serious AEs were reported in 3% (11 participants) [58] . In another open extension study in a subset of 229 of 886 original participants, nearly 5% experienced serious AEs [59] .
In this systematic review and meta-analysis we evaluated whether newer drugs for the treatment of BPH associated LUTS offered advantages over established treatments, primarily older ABs (ie, tamsulosin, alfuzosin, doxazosin). Our principal finding was that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH were more effective compared with older AB monotherapy. Further, AEs with newer treatments or combinations were similar or greater than those for older AB monotherapy when evidence was sufficient to assess.
Strengths of this review include its rigorous methodology that followed a written, a priori protocol that was developed according to AHRQ standards. We focused on the analysis of comparative effectiveness with prespecified outcomes addressing treatment effectiveness and potential harms, which are equally important to patients considering these treatments. Lastly, we used a well-established and comprehensive approach to determine the SOE beyond study limitations alone that included domains such as inconsistency and imprecision. Although our focus on English language publications is a potential limitation, we were reassured by the finding that supplemental searching of www.clinicaltrials.gov , which requires registration of drug trials subject to FDA administration did not identify any additional publications for trials within 2 yr of completion. We therefore do not believe that the results of this study were affected by language bias. Weaknesses of this study largely relate to the limitation of the body of evidence that we critically reviewed. Few comparisons were assessed as high SOE in our review as the result of study limitations (for example lack of blinding), imprecision, and heterogeneity. Important outcomes relating to potential treatment-related harms such as disease progression leading to AUR and/or surgical intervention could not be evaluated due to the short duration of all eligible RCTs and a paucity of observational studies and their limited quality. Given that LUTS attributed to BPH is a chronic and progressive condition, available evidence provided little insight about the relative long-term effects of treatments including prevention of symptom progression, development of AUR, or need for surgical or other interventions.
Several recently published systematic reviews have sought to address the body of evidence on drug therapy for LUTS attributed to BPH, but these either have not been comprehensive or lacked a rigorous assessment of the evidence quality beyond study design. Most notably, the European Urology Association guidelines were based on a systematic review that used the 2011 version of the Oxford Center for Evidence-Based Medicine levels of evidence [60] . As a result, all evidence from meta-analyses of RCTs or individual trials were labeled as Level 1 evidence, which we perceive to be misleading. A systematic review by Navara et al [61] used the same levels of evidence rating system and focused on the newer AB silodosin, which it found as effective as tamsulosin 0.4 mg yet with fewer AEs except for abnormal ejaculation. Based on moderate SOE, our analyses agreed with findings of similar comparative effectiveness, but raised concerns about a less favorable side-effect profile. With regards to phosphodiesterase type-5 inhibitors (PDE-5 inhibitors), two systematic reviews published in urology literature failed to rate the quality of evidence and are therefore of limited value in informing evidence-based clinical practice [62 63] . The most rigorous assessment of newer drugs for LUTS attributed to BPH was conducted as part of the National Institute for Health and Care Excellence guideline published in 2010 with evidence updates added through 2014. While their evidence update references newer trial evidence and other systematic reviews, they did not conduct their own updated analysis, thereby resulting in an evidence report lacking information on PDE-5 inhibitors, anticholinergics, and mirabegron. Our study therefore appears timely and makes an important contribution for patients, providers, and health policy makers seeking to assess the relative merits of newer agents for treating male LUTS.
Some of the newer drugs included in this review such as silodosin should be viewed as offering alternative treatment options of possibly similar efficacy to existing agents rather than superior management options, although often with potentially greater harms. PDE-5 inhibitors may offer conceptual advantages in patients suffering from both ED and LUTS. Other new drugs appear either less effective or the evidence for assessing their effectiveness was insufficient. It was notable that we only identified three eligible trials of 5-ARIs. Given the well-defined track record of established agents and their usually lower costs, existing evidence supports older ABs and/or 5-ARIs as initial pharmacologic options. Trials of antimuscarinics, which are known to affect bladder contractility, often excluded participants with higher postvoid residual urine volumes, thereby excluding patients at highest risk for urinary retention and affecting the applicability of their findings to general medical practice in which an assessment of postvoid residuals is not part of the routine care pathway.
Additional research would add valuable information on the treatment of LUTS attributed to BPH. Most trials we identified had a time horizon of 3 mo or less; trials with a longer treatment duration that reflect real-life practice would provide valuable information to assess durability of effect, prevention of progression including risk of AUR and need for surgical intervention, as well as long-term compliance and harms. Although we found little benefit of the newer drugs compared with or added to older ABs overall, it is possible that they provide benefits to select groups of patients. However, we identified few trials that examined effects within our prespecified subgroups; available analyses were posthoc, limiting the validity and reliability of the evidence.
Our study found that none of the drugs or drug combinations newly used to treat LUTS attributed to BPH was more effective compared with older AB treatment. AEs of the new drugs or drug combinations were similar or greater than older ABs when AE evidence was sufficient to assess. Evidence was generally insufficient to assess long-term efficacy, prevention of symptom progression, or AEs. Given the lack of superior effectiveness, less assurance of their relative safety and likely greater cost, the value of newer drugs alone or in combination for treating LUTS attributable to BPH appears low.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dahm, Brasure, Risk, Fink, Wilt.
Acquisition of data: Brasure, MacDonald, Olson, Nelson, Rwabasonga.
Analysis and interpretation of data: Dahm, Brasure, Risk, Fink, Wilt.
Drafting of the manuscript: Dahm, Brasure, MacDonald, Risk, Fink, Wilt.
Critical revision of the manuscript for important intellectual content: Dahm, Brasure, Risk, Fink, Wilt.
Statistical analysis: Dahm, MacDonald, Wilt.
Obtaining funding: Wilt.
Administrative, technical, or material support: Brasure, Nelson.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This project was funded under Contract Number HHSA290201200016I from the Agency for Healthcare Research and Quality, US Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Agency for Healthcare Research and Quality or the US Department of Health and Human Services. The Agency for Healthcare Research and Quality retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency's contract with the author.
Funding/Support and role of the sponsor: This reported is based on research conducted by the Minnesota Evidence-based Practice Center under contract with the Agency for Healthcare Research and Quality, Rockville, Maryland (Contract Number: HHSA290201200016I).