Background
Laparoscopic and robotic simple prostatectomy (SP) have been introduced with the aim of reducing the morbidity of the standard open technique.
Objective
To report a large multi-institutional series of minimally invasive SP (MISP).
Design, setting, and participants
Consecutive cases of MISP done for the treatment of bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) between 2000 and 2014 at 23 participating institutions in the Americas and Europe were included in this retrospective analysis.
Intervention
Laparoscopic or robotic SP.
Outcome measurements and statistical analysis
Demographic data and main perioperative outcomes were gathered and analyzed. A multivariable analysis was conducted to identify factors associated with a favorable trifecta outcome, arbitrarily defined as a combination of the following postoperative events: International Prostate Symptom Score 15 ml/s, and no perioperative complications.
Results and limitations
Overall, 1330 consecutive cases were analyzed, including 487 robotic (36.6%) and 843 laparoscopic (63.4%) SP cases. Median overall prostate volume was 100 ml (range: 89–128). Median estimated blood loss was 200 ml (range: 150–300). An intraoperative transfusion was required in 3.5% of cases, an intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3%. Median length of stay was 4 d (range: 3–5). On pathology, prostate cancer was found in 4% of cases. Overall postoperative complication rate was 10.6%, mostly of low grade. At a median follow-up of 12 mo, a significant improvement was observed for subjective and objective indicators of BOO. Trifecta outcome was not significantly influenced by the type of procedure (robotic vs laparoscopic; p = 0.136; odds ratio [OR]: 1.6; 95% confidence interval [CI], 0.8–2.9), whereas operative time (p = 0.01; OR: 0.9; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.9; 95% CI, 0.9–1.0) were the only two significant factors. Retrospective study design, lack of a control arm, and limited follow-up represent major limitations of the present analysis.
Conclusions
This study provides the largest outcome analysis reported for MISP for BOO/BPE. These findings confirm that SP can be safely and effectively performed in a minimally invasive fashion in a variety of healthcare settings in which specific surgical expertise and technology is available. MISP can be considered a viable surgical treatment in cases of large prostatic adenomas. The use of robotic technology for this indication can be considered in centers that have a robotic program in place for other urologic indications.
Patient summary
Analysis of a large data set from multiple institutions shows that surgical removal of symptomatic large prostatic adenomas can be carried out with good outcomes by using robot-assisted laparoscopy.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
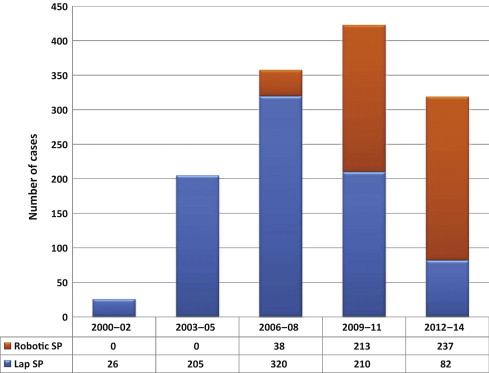
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).
Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Open simple prostatectomy (OSP) has represented the standard surgical treatment for bladder outlet obstruction (BOO) due to benign prostatic enlargement (BPE) for a century, and it is still recommended as a standard therapy for large (>80-ml) glands [1] and [2]. However, this procedure can be associated with a high risk of perioperative complications and prolonged hospitalization times [3], [4], and [5]. Thus, alternative minimally invasive options have been introduced and implemented with the aim of reducing surgical morbidity. These alternatives are chiefly laser technology–based procedures [6] and [7] and bipolar technology–based procedures [8] and [9].
Laparoscopic simple prostatectomy (LSP) was first described in 2002 by Mariano et al [10] and has since been reported by several investigators with encouraging outcomes [11] . In 2008, the feasibility of robot-assisted simple prostatectomy (RASP) was demonstrated by Sotelo et al [12] , and the procedure was subsequently embraced by others because of the potential advantages compared with the standard open procedure [13] . A recent meta-analysis including 27 studies with 764 patients confirmed that minimally invasive simple prostatectomy (MISP; laparoscopic and robotic) provides improvements similar to those of OSP, with a longer operative time but less blood loss and shorter hospital stay [14] . Nevertheless, these two minimally invasive procedures remain to be further scrutinized, as all the reported data are from series with limited samples, mostly from single centers.
The aim of this study is to provide an outcome analysis of a large multi-institutional series of MISP and to identify factors associated with a favorable surgical outcome.
Consecutive cases of MISP performed between 2000 and 2014 at 23 participating institutions in the Americas (United States, Venezuela, Brazil, and Chile) and Europe (France, Italy, Portugal, Poland, Belgium, Turkey, and Sweden) were included in this retrospective study. Each group performed the procedure according to its own surgical indication, technique, and follow-up protocol. Raw data without any identifiers were gathered into a standardized data sheet, which was specifically built for study purposes. Each center had either an institutional review board approval or a waiver in place for this study. Study coordinators were the first and senior authors of this report.
Table 1 gives an overview of the surgical techniques. Prophylactic antibiotics and thromboembolic preventive therapies had been adopted in all centers. A Trendelenburg position with different degrees of angulation was used.
Table 1 Surgical techniques in minimally invasive simple prostatectomy: an overview
| Procedure | Technical nuances |
|---|---|
| Laparoscopic transcapsular | Midline incision covering the anterior aspect of the prostatic capsule and bladder neck; early vascular control; prostate morcellation [10] |
| Transversal incision on the prostate capsule; adenoma dissected with scissors or Maryland forceps [21] | |
| Adenoma enucleation by finger assistance [18] and [19] | |
| Laparoscopic transvesical | Transverse cystostomy; adenoma dissection with J hook cautery and suction–irrigation cannula [22] |
| Transverse cystostomy; adenoma dissection with harmonic scalpel and suction–irrigation cannula [20] | |
| Robotic transcapsular | Transverse capsular incision approximately 1–2.5 cm from the vesicoprostatic junction [24] and [29] |
| Robotic transvesical | Horizontal cystotomy proximal to the vesicoprostatic junction; stitches placed in the lateral lobes to create traction during the adenoma dissection [12], [26], and [29] |
| Ligation of DVC; incision of the anterior bladder neck just proximal to the vesicoprostatic junction; adenoma dissection started posteriorly; plication of the posterior prostatic capsule, continuous vesicourethral anastomosis, anterior prostatic capsule sutured to the anterior bladder wall [23] | |
| Transverse cystotomy at the bladder neck; traction suture placed on the adenoma; use of a robotic tenaculum grasper [25] | |
| Bladder dome identified and midline cystotomy made; stay sutures to keep the edges of the cystotomy open; traction suture through the adenoma to aid dissection [27] | |
| Intrafascial procedure: early release of neurovascular bundles; DVC suture ligated; horizontal cystotomy incision at the bladder neck; seminal vesicles transected at the prostate base, vesicourethral anastomosis [28] |
DVC = dorsal vein complex.
The LSP was performed using different personal techniques developed based on the principles of transcapsular (Millin) [15] , transvesical (Freyer) [16] , or transvesicocapsular (Bourque) [17] approaches described for OSP. Each investigator adopted specific intraoperative strategies and technical nuances to optimize the procedure [10], [18], [19], [20], [21], and [22].
RASP was also performed using a variety of techniques based on the same technical principles [12], [23], [24], [25], [26], [27], [28], and [29]. In one center, an intrafascial technique was selectively used [28] .
Demographic data, including age, body mass index (BMI), Charlson comorbidity score, and past surgical history, were gathered. Baseline outcome parameters for BOO were also recorded; they included the International Prostate Symptom Score (IPSS) (and quality of life score), maximum flow rate (Qmax), prostate-specific antigen (PSA), and Sexual Health Inventory for Men (SHIM) score. Other relevant disease-specific parameters included the presence of an indwelling Foley catheter, associated bladder disease (stones or diverticula), prostate volume, and adenoma volume. Main features of the surgical techniques were also accrued.
The following outcome parameters were analyzed: operative time, estimated blood loss, intraoperative adverse events (grade according to Satava [30] ), conversions, length of stay, hemoglobin level drop, time to Foley removal, and time to drain removal. Pathology findings were assessed. Both medical and surgical complications occurring ≤90 d after surgery were captured, including inpatient stay and outpatient setting, and these were graded according to the standardized Clavien-Dindo system [31] .
Postoperative outcomes specifically related to the treatment of BOO were recorded, including PSA, Qmax, and IPSS. The SHIM (International Index of Erectile Function-5) score was used to estimate the impact of the procedures on sexual function. Follow-up was calculated from the date of surgery to the date of the most recent documented examination. In each center, a variable follow-up protocol including an assessment of IPSS, Qmax, postvoid residual (PVR), and PSA was adopted.
Patients’ baseline characteristics were reported as frequencies (percentages) and median and interquartile range for categorical and continuous variables, respectively. Preoperative and postoperative continuous variables were compared using the Wilcoxon matched-pairs signed rank test. A multivariable analysis was conducted to determine the factors predictive of composite (ie, trifecta) favorable outcome, which was arbitrarily defined as a combination of the following items: (1) no perioperative complications, (2) postoperative IPSS <8, and (3) postoperative Qmax >15 ml/s. The following clinically relevant parameters were included in the multivariable model: age at surgery, BMI, prostate gland volume, operative time, estimated blood loss, previous abdominal surgery, and technique (laparoscopic or robotic). The model was also controlled for the presence of a center in which the original laparoscopic technique was developed [10] ; that center represented an outlier in terms of surgical expertise with the procedure, as it had provided >250 cases, thus being far beyond the learning curve.
On multivariate analysis, results are expressed as odds ratios (ORs) with their 95% confidence intervals (CIs). For all statistical analyses, a two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS v.21 software (IBM Corp., Armonk, NY, USA).
A total of 1330 cases were done at participating institutions over the study period. Most of the procedures were done laparoscopically (n = 843; 63.4%), but there was an increase in the number of robotic cases over time ( Fig. 1 ).

Main patient characteristics are summarized in Table 2 . Patients presented with a baseline median IPSS of 23 (range: 20–31) and a median Qmax of 5 ml/s (range: 5–8). Median prostate volume was 100 ml (range: 89–128). In 12.9% of cases, patients had an indwelling Foley catheter. In 1.6% of cases, a bladder diverticulum requiring excision was recorded.
Table 2 Overview of study population, procedures, and techniques (n = 1285)
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Age, yr, median (IQR) | 66 (62–72) | 65 (62–71) | 67 (63–73) |
| BMI, kg/m2, median (IQR) | 26 (24–28) | 25 (24–27) | 27.3 (25–30) |
| Charlson index, median (IQR) | 2 (1–4) | 4 (2–5) | 2 (1–2.5) |
| Prior abdominal/pelvic surgery, no. (%) | 197 (14.8) | 80 (9.5) | 117 (24) |
| Baseline IPSS, median (IQR) | 23 (20–31) | 24 (20–32) | 23 (18–27) |
| Baseline QoL, median (IQR) | 5 (4–6) | 5 (4–6) | 4 (3–5) |
| Baseline Qmax, ml/s, median (IQR) | 5 (5–8) | 5 (5–6) | 8 (5–11) |
| Baseline PSA, median (IQR) | 6.5 (4.2–9.5) | 7 (5–10) | 6.2 (3.7–11) |
| Baseline PVR, ml, median (IQR) | 150 (90–194) | 180 (95–190) | 108 (55–231) |
| Baseline SHIM score, median (IQR) | 16 (9–22) | 19 (10–25) | 15 (9–21) |
| Baseline Hb, mg/dl, median (IQR) | 14.8 (13.4–15.6) | 15 (15–16) | 14 (12.9–15) |
| Indwelling Foley, no. (%) | 172 (12.9) | 70 (8.3) | 102 (20.9) |
| Preoperative prostate biopsy, no. (%) | 184 (13.8) | 78 (9.2) | 106 (21.7) |
| Bladder stones, no. (%) | 45 (3.4) | 30 (3.5) | 15 (3) |
| Bladder diverticula, no. (%) | 21 (1.6) | 13 (1.5) | 8 (1.6) |
| Prostate volume, ml, median (IQR) | 100 (89–128) | 99 (89–121) | 110 (86–140) |
| Adenoma volume, ml, median (IQR) | 70 (56–90) | 61 (48–79) | 80 (65–97) |
BMI = body mass index; Hb = hemoglobin; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; PSA = prostate-specific antigen; PVR = postvoid residual; Qmax = maximum flow rate; QoL = quality of life; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Main surgical outcomes are described in Table 3 . A transperitoneal approach was mostly used in the robotic series, whereas an extraperitoneal approach was used for most laparoscopic cases (p < 0.001). Median operative time was 100 min, and median estimated blood loss was 200 ml. An intraoperative transfusion was required in 3.5% of cases, and the median hemoglobin level drop on postoperative day 1 was 2 (range: 1–3.2) mg/dl. Median length of stay was 4 d (range: 3–5), whereas median time to Foley removal was 5 d (range: 4–7) d. On pathology, a prostate cancer was found in 4% of cases. An intraoperative complication was recorded in 2.2% of cases, and the conversion rate was 3% (2.9% for LSP and 3.1% for RASP). The postoperative complication rate was 10.6% (7.1% for LSP and 16.6% for RASP), most of complications being of low grade (Clavien 1–2) ( Table 4 ).
Table 3 Surgical outcomes
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Technique, no. (%) | |||
| Millin | 1041 (78.2) | 735 (87.2) | 306 (62.8) |
| Others | 289 (21.8) | 108 (12.8) | 181 (37.2) |
| Approach, no. (%) | |||
| Transperitoneal | 494 (37.1) | 104 (12.4) | 390 (80) |
| Extraperitoneal | 836 (62.9) | 739 (87.6) | 97 (20) |
| ORT, min, median (IQR) | 100 (90–150) | 95 (85–120) | 154.5 (100–180) |
| EBL, ml, median (IQR) | 200 (150–300) | 280 (150–300) | 200 (100–400) |
| Hemoglobin POD1, mg/dl, median (IQR) | 12 (12–13) | 12 (11–13) | 12 (11–13) |
| Time to Foley removal, median (IQR) | 5 (4–7) | 4 (4–6) | 7 (5–9) |
| Time to drain removal, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) |
| Length of stay, d, median (IQR) | 4 (3–5) | 4 (3–5) | 2 (1–4) |
| Pathology, no. (%) | |||
| BPH | 1255 (94.4) | 815 (96.7) | 440 (90.3) |
| Adenocarcinoma | 53 (4) | 15 (1.7) | 38 (7.8) |
| HGPIN/ASAP | 20 (1.5) | 12 (1.4) | 8 (1.6) |
| Others | 2 (0.1) | 1 (0.1) | 1 (0.2) |
| Specimen weight, g, median (IQR) | 75 (62–100) | 76 (67–97) | 75 (52–101) |
| Postoperative PSA, ng/dl, median (IQR) | 1.1 (0.6–1.9) | 1 (1–2) | 1.1 (0.5–2) |
| Postoperative Qmax, ml/s, median (IQR) | 22 (20–27) | 22 (20–26) | 25 (20–33) |
| Postoperative IPSS, median (IQR) | 4 (2–5) | 5 (3–5) | 7 (4–9) |
| Postoperative SHIM, median (IQR) | 17 (9–23) | 20 (12–25) | 15 (8–21) |
BPH = benign prostatic hyperplasia; EBL = estimated blood loss; HGPIN/ASAP = high-grade prostate intraepithelial neoplasia/atypical small acinar proliferation; IPSS = International Prostate Symptom Score; IQR = interquartile range; LSP = laparoscopic simple prostatectomy; ORT = operative time; POD = postoperative day; PSA = prostate-specific antigen; Qmax = maximum flow rate; RASP = robot-assisted simple prostatectomy; SHIM = Sexual Health Inventory for Men.
Table 4 Perioperative complications
| Overall (n = 1330) | LSP (n = 843) | RASP (n = 487) | |
|---|---|---|---|
| Intraoperative complications, ∧ no. (%) | |||
| Grade 1 | 14 (1) | 4 (0.5) | 11 (2.2) |
| Grade 2 | 15 (1.1) | 10 (1.2) | 5 (1) |
| Overall | 29 (2.2) | 14 (1.7) | 15 (3.2) |
| 90-d postoperative complications, * no. (%) | |||
| Grade 1 | 74 (5.5) | 42 (5) | 32 (6.5) |
| AUR (requiring catheterization) | 26 | 16 | 10 |
| Ileus (requiring antiemetics, bowel rest) | 13 | 10 | 3 |
| Hematuria/clots (requiring catheter irrigation) | 19 | 6 | 13 |
| ED (requiring phosphodiesterase inhibitor) | 4 | 4 | 0 |
| Urgency/incontinence (requiring anticholinergics) | 5 | 1 | 4 |
| Others¶ | 6 | 5 | 1 |
| Grade 2 | 50 (3.3) | 13 (0.6) | 37 (8) |
| UTI (requiring antibiotics) | 30 | 10 | 20 |
| Anemia (requiring transfusions) | 7 | 2 | 5 |
| Wound infection | 2 | 0 | 2 |
| DVT/PE | 2 | 0 | 2 |
| Others¥ | 9 | 1 | 8 |
| Grade 3a | 15 (1.1) | 5 (0.6) | 10 (2) |
| Urethral/bladder neck stricture (requiring endoscopy) | 6 | 3 | 3 |
| Hematuria/clots (requiring endoscopy) | 4 | 1 | 3 |
| Urinary fistula (requiring surgical repair) | 2 | 1 | 1 |
| Wound infection (requiring surgical repair) | 1 | 0 | 1 |
| Hem-o-Lok migration (requiring endoscopy) | 1 | 0 | 1 |
| Bleeding hypogastric artery (requiring embolization) | 1 | 0 | 1 |
| Grade 4 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Cardiac heart failure | 1 | 0 | 1 |
| Grade 5 | 1 (0.1) | 0 (0) | 1 (0.2) |
| Incarcerated hernia/sepsis/multiorgan failure/death | 1 | 0 | 1 |
| Overall | 141 (10.6) | 60 (7.1) | 81 (16.6) |
∧ Graded according to Satava [30] .
* Graded according to Clavien-Dindo [31] . ¶ Others in grade 1 include pain, fever, wound discharge, anastomotic leakage, and stress incontinence. ¥ Others in grade 2 include dyspnea, diarrhea positive for Clostridium difficile, epididymitis, fever, paravesical abscess, pneumonia, and retained Foley.
AUR = acute urinary retention; DVT/PE = deep vein thrombosis/pulmonary embolism; ED = erectile dysfunction; LSP = laparoscopic simple prostatectomy; RASP = robot-assisted simple prostatectomy; UTI = urinary tract infection.
At a median follow-up of 12 mo, a significant improvement was observed for Qmax (p < 0.001) and IPSS (p < 0.001). There was a significant decline in the PSA values postoperatively (p < 0.001), whereas no significant change was noted in terms of SHIM score (p = 0.90).
At multivariable analysis, age (p = 0.89), BMI (p = 0.23), prostate volume (p = 0.68), and history of previous surgery (p = 0.18) were not significant factors predictive of a favorable trifecta outcome. Similarly, the outcome was not significantly different when considering the type of procedure (robotic vs laparoscopic) (p = 0.13; OR: 1.6; 95% CI, 0.8–2.9). Operative time (p = 0.01; OR: 0.99; 95% CI, 0.9–1.0) and estimated blood loss (p = 0.03; OR: 0.99; 95% CI, 0.9–1.00) were the only two significant factors associated with higher likelihood of obtaining a favorable outcome. Besides these factors, the fact of having performed a remarkably high number of cases (which was the case for one outlier participating center) represented a highly significant predictive factor (p < 0.001; OR: 0.8; 95% CI, 0.4–0.1).
Current guidelines still indicate that OSP is an effective and durable procedure for the treatment of benign prostatic hyperplasia–lower urinary tract symptoms among men with a large prostate volume (>80 ml) [1] and [2]. However, OSP is also a relatively invasive surgical procedure, given the high morbidity and complication rates [3], [4], and [5]. With the aim of combining the outcomes of OSP with the minimal invasiveness of standard and robot-assisted laparoscopy, several groups have explored the option of MISP. However, the role of this procedure is still under scrutiny in the current era of rapid advances of laser technology–based techniques.
In this paper we report the largest series of MISPs to date. The present analysis provides an overview of practice patterns, techniques, and outcomes of MISP in several major centers in Europe, North America, and South America that have pioneered and implemented these procedures. The outcomes of this study corroborate the findings of three recent systematic reviews on LSP and RASP. Asimakopoulos et al reported a critical analysis of the current literature on the role of LSP [11] . Fourteen studies (11 case series and 3 comparative retrospective nonrandomized case–control studies) were included, with a total of 626 patients treated. Compared with OSP, LSP was found to be associated with less blood loss, a shorter postoperative catheterization period, and shorter hospital stay, but at the expense of a longer operative time. Banapour et al systematically reviewed the evidence regarding RASP outcomes and identified eight published studies, all noncomparative case series [29] . A total of 109 RASP cases were included in their analysis. In all these series, a substantial postoperative improvement in urinary symptoms was observed, suggesting that RASP is safe and effective. More recently, Lucca et al [14] looked at 27 observational studies with 764 patients and concluded that MISP seems to be an effective and safe treatment option. However, the authors pointed out the need for prospective randomized studies comparing OSP, MISP, and laser enucleation.
Our study population mirrors those reported in other studies in this setting, featuring severely symptomatic patients (median IPSS: 5; median Qmax: 5 ml/s; median PVR: 150 ml) with large prostate glands (median prostate volume: 100 ml).
It is not surprising that the role of robot-assisted laparoscopic surgery has expanded for this indication over the years in these institutions, as shown in Figure 1 . Simple prostatectomy is a demanding procedure when done in a minimally invasive fashion, as it includes challenging extirpative steps (adenoma dissection) and reconstructive steps (hemostasis of the prostate bed, retrigonization, and bladder suturing). As in more common urologic procedures, such as radical prostatectomy [32] or pyeloplasty [33] , the addition of robotic technology in simple prostatectomy can be regarded as a helpful tool for physicians embarking on this surgical endeavor. Each surgeon has personalized the procedure with specific operative strategies. Robotic procedures have been mostly done through a transperitoneal approach, which reflects the experience with radical prostatectomy [34] . In contrast, laparoscopic procedures have been carried out mostly with an extraperitoneal approach, again reflecting experience matured with laparoscopic radical prostatectomy [35] and [36]. The dissection of the adenoma can be facilitated by finger assistance, placement of a retraction suture on the adenoma, or use of specific instruments (ie, the robotic tenaculum). Hemostasis in laparoscopic cases has been achieved with selective suturing of bleeders in the prostatic resection bed [18], [19], and [21]. With the adoption of robot-assisted surgery, more complex suturing tasks have been explored, such as plication of the prostatic capsule or vesicourethral anastomosis [23] and [28].
When put in the perspective of reported outcomes of other surgical procedures, the analysis of outcomes in the present large series provides a few points deserving attention ( Table 5 ). The procedure can be carried out within acceptable operative times, especially considering that the learning phase of the participating centers is included in this paper. Operative times appear to be longer than reported for Holmium laser enucleation of the prostate [6] and [7] or for OSP [3] and [4], especially for RASP. The estimated blood loss seems to be lower than reported for OSP [3] and [4]; this situation is also reflected in the low intraoperative transfusion rate (3.5%), which compared favorably with the rates seen for laser techniques for similar-sized glands [6] and [7]. In terms of hospital stay, MISP techniques, especially RASP, seem to offer shorter times compared with those reported in contemporary series of OSP [3] and [4], but in this regard, laser and bipolar techniques are more attractive [6], [7], [8], and [9]. Similarly, catheterization time seems to be in favor of transurethral procedures; this can be regarded as a sensitive issue for the patient, as it directly affects his quality of life in the immediate postoperative period.
Table 5 Overview of surgical outcomes of procedures for bladder outlet obstruction due to large prostatic adenoma
| Study | Procedure | Cases, no. | OT, min | Hb drop, mg/dl | Hospital stay, d |
Transfusion rate, % | Catheter time, d |
Short-term postoperative complication rate, % |
|---|---|---|---|---|---|---|---|---|
| Serretta et al [3] | OSP | 1804 | NA | NA | 7 | 8.2 | 5 | 37.1 |
| Gratzke et al [4] | 902 | 80.8 * | NA | 11.9 * | 7.5 | NA | 17.3 | |
| Kuntz et al [6] | HoLEP | 60 | 135.9 * | 1.9 * | 1.3 * | NA | 2.9 * | 15 |
| Humphreys et al [7] | 117 | 116.5 * | NA | 1 * | NA | 0.6 * | 10.2 | |
| Geavlete et al [8] | Bipolar TUR | 70 | 91.4 * | 1.7 * | 2.1 * | NA | 1.5 * | 21.4 * |
| Chen et al [9] | PKEP | 80 | 121.2 ∧ | 1 ∧ | 3 ∧ | NA | 1.6 ∧ | 27.5 ∧ |
| Present series | MISP | 1330 | 100 ∧ | 2.8 ∧ | 4 ∧ | 3.5 | 5 ∧ | 10.6 ∧ |
* Mean values.
∧ Median values.
Hb = hemoglobin; HoLEP = holmium laser enucleation of the prostate; MISP = minimally invasive simple prostatectomy; na = not available; OSP = open simple prostatectomy; OT = operative time; PKEP = plasmakinetic enucleation of the prostate; TUR = transurethral resection.
We found a low postoperative complication rate, and most of the complications were low grade (Clavien 1–2), which translates into minimal clinical impact on the regular postoperative course. As with any other surgical treatment for BOO/BPE [37] and [38], MISP should also be assessed on the basis of changes in subjective parameters of obstruction (IPSS) and objective parameters (flow rate and Qmax). In this respect, a highly significant improvement was recorded after 1 yr of follow-up. In addition, a significant decline in PSA levels was recorded, and this report represents an indirect proof of the efficacy of the treatment [39] because MISP allows complete enucleation of the adenoma, duplicating the established principles of the open procedure. The median pathology specimen weight in our series was 75 g. The SHIM score in the study population remained stable after surgery, which can be interpreted as a minimal impact of the MISP procedure on sexual function. These findings demonstrate that MISP is a safe, reproducible, and effective surgical treatment option for BOO/BPE. An additional advantage of performing MISP is the ability to treat concomitant bladder conditions, such as bladder stones or diverticula. This was the case for a small subgroup of patients in our population.
The selection of one technique over the others is influenced by a variety of factors, including surgical expertise and experience, instrument availability, cost issues, and type of clinical practice.
The strength of the present analysis is represented by the large sample. The use of a purpose-built centralized reporting system afforded a real-life data set, which can enjoy a higher external validity than more formal analyses. Nevertheless, the present analysis has limitations. First, the retrospective study design carries some intrinsic biases, including possible underreporting of data. The analysis was limited to the variables that were available and of sufficient quality to allow a reliable assessment. As mentioned, each group performed the procedure according to its own surgical indication, technique, and follow-up protocol, which translates into a lack of standardization. Second, most of the participating centers had already partially reported their own single-center experiences over the last decade. It should be noted that we are unable to provide a specific analysis of the surgical background of each participating center/surgeon. However, all these institutions have in place well-established laparoscopic and/or robotic programs, and they are well recognized as high-volume institutions for minimally invasive surgery. Third, no control group was considered in the study design, thus not allowing a comparison with other established techniques for the treatment of BOO/BPE that could have been adopted in each center over the study period. In addition, we did not perform a head-to-head comparison of laparoscopy compared with robotic surgery. Again, this was not in the scope of this analysis because of inherent biases. However, the type of technique was included in the multivariable model to determine its ultimate effect on the outcomes. Ideally, a matched comparison could be done on this same data set for a later comparative analysis.
We decided to use a composite outcome, defined as trifecta, to have a marker of surgical quality for the simple prostatectomy procedure. During the preparation of the manuscript, different ways of defining the trifecta were considered, given the lack of a reference in the present literature for such an outcome. Thus, this parameter was arbitrarily chosen, and this fact can be considered a limitation. The trifecta, as defined in this paper, would ideally require external validation in other series.
The follow-up of this series still remains limited and therefore suboptimal. A long-term outcome analysis is needed to allow a comparison with more established surgical procedures for BOO/BPE [37] and [38]. Cost related to minimally invasive technologies, especially robotic technology, remains a highly debated issue [40] . A formal cost analysis was not within the scope of this analysis, given the fact that hospital costs and reimbursement vary significantly in different countries and healthcare systems. One might point out that with the laparoscopic procedure, instrument-related cost is minimal, in contrast with the robotic procedure, in which instrumentation implies a higher cost; however, this cost can be depreciated if a robotic program is already in place (for other, more common indications), as maximizing the use of the robotic system might have favorable economic implications. Matei et al found the costs of RASP to be lower than those of OSP, and to be equivalent to those of transurethral resection of the prostate [26] . Sutherland et al reported the cost of RASP to be expectedly high compared with OSP, adding an average of $2797 to the operating charges [24] . Further investigation is needed in this area.
We report the largest series of MISP for BOO/BPE. Despite recognized limitations of this analysis (retrospective study design, lack of control group, limited follow-up, and lack of cost analysis), the present findings confirm that simple prostatectomy can be safely and effectively performed in a minimally invasive fashion using a standard or robot-assisted laparoscopic approach in a variety of healthcare settings. The use of robotic technology for this specific indication can be considered in centers at which a robotic program is already in place for other common urologic indications. Ultimately, the role of MISP in the surgical armamentarium for BPE warrants further clinical investigation.
Author contributions: Riccardo Autorino had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Autorino, Porpiglia.
Acquisition of data: Pini, Nuñez Bragayrac, Varkarakis, Amparore, Ferro, Gallo, Nething, Chopra, N. Patel, Champ Weeks, Spier, Kowalczyk, Lynch, Thiel, Harbin, Dias, Verghese, Samavedi, Molina, Ahallal.
Analysis and interpretation of data: Autorino, Zargar.
Drafting of the manuscript: Autorino.
Critical revision of the manuscript for important intellectual content: Mariano, Sanchez-Salas, Sotelo, Chlosta, Castillo, Matei, Celia, Koc, Vora, Aron, Parsons, Jensen, Sutherland, Cathelineau, Hwang, Derweesh, Volpe, Vuruskan, Bandi, Muruve, Laydner, Cherullo, Haber, Kaouk, De Cobelli, Lagerkvist, Kim, Lima, V. Patel, White, Mottrie.
Statistical analysis: Zargar
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Porpiglia.
Other (specify): None.
Financial disclosures: Riccardo Autorino certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.