Context
Lower urinary tract symptoms (LUTS) are one of the most common and troublesome nonmalignant conditions affecting quality of life in aging men. A spectrum of established medical and surgical options is available to provide relief of bothersome LUTS. Both the adverse events of medication and the morbidity with surgical treatment modalities have to be counterbalanced against efficacy. Novel minimally invasive treatment options aim to be effective, ideally to be performed in an ambulatory setting under local anaesthesia and to offer a more favourable safety profile than existing reference techniques.
Objective
A comprehensive, narrative review of novel minimally invasive treatment modalities for the management of male LUTS due to benign prostatic enlargement is presented.
Evidence acquisition
Medline, PubMed, Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to benign prostatic enlargement.
Evidence synthesis
With regard to newly devised intraprostatic injectables (botulinum neurotoxin A, NX1207, PRX302), PRX302 is currently the only substance that was superior to placebo in a phase 3 RCT providing proof of efficacy and safety. The prostatic urethral lift technique has been evaluated in several phase 3 trials showing rapid and durable relief of LUTS without compromising sexual function in carefully selected patients without a prominent median lobe. The first clinical experience of the temporary implantable nitinol device demonstrated that implantation of this novel device is a safe procedure, easy, and fast to perform. Further studies are required to evaluate efficacy, durability, and to define appropriate patient selection. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are in the early stages of development. Prostatic artery embolization performed by interventional radiologists at specialised centres shows a high technical success rate in the treatment of bothersome LUTS. However, a substantial clinical failure rate and a particular spectrum of complications not commonly seen after urologic interventions do occur and need to be critically evaluated.
Conclusions
Initial promising clinical results on novel minimally invasive treatment options indicate efficacy comparable to standard techniques, often associated with a more favourable safety profile, in particular with preservation of sexual function. Many of these techniques are in their infancy and based on experience of new developments in the past. Further RCTs are required to evaluate efficacy, safety, and durability of novel techniques with long-term follow-up and careful evaluation of the selection criteria, which have been applied in clinical trials. The prostatic urethral lift is the only procedure with Level 1 evidence data and that can therefore be recommended for treatment of male LUTS in clinical practice for selected patients.
Patient summary
Minimally invasive treatment options have been developed to provide relief of lower urinary tract symptoms comparable to standard surgical techniques with a more favourable safety profile. However, long-term clinical evaluation is still needed for most of these innovations before they can be recommended to be an effective replacement for standard surgical treatment.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.
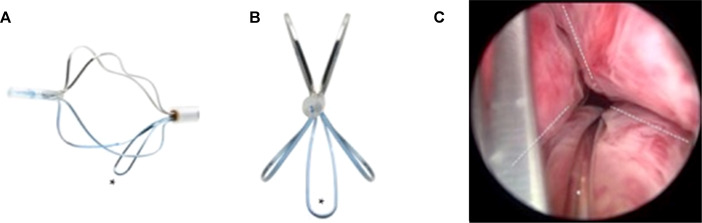
Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.
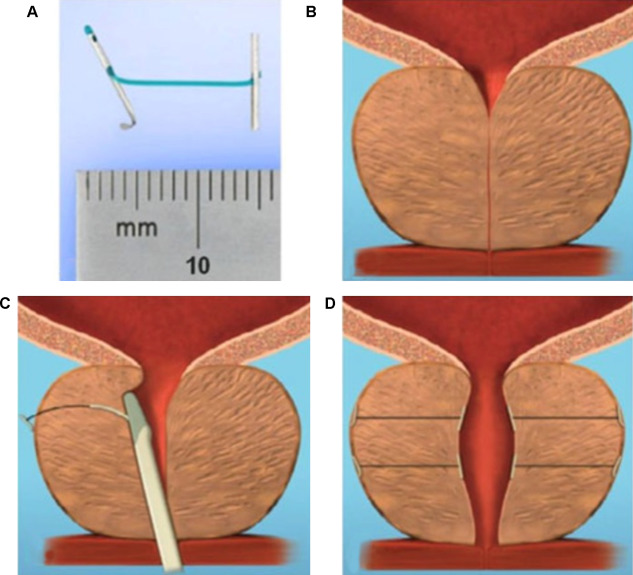
Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .
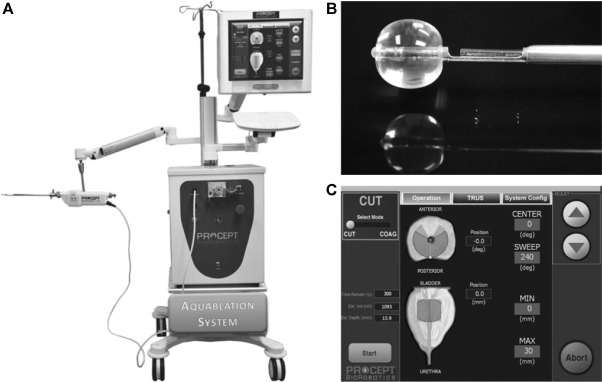
Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.
Lower urinary tract symptoms (LUTS) considerably impair quality of life in men. LUTS represent one of the most common nonmalignant conditions with significant socio-economic importance to public health systems worldwide. Male LUTS due to benign prostatic enlargement (LUTS/BPE) is the most common reason next to urinary tract infections for urologic consultation in clinical practice [1] . Annual expenditures on the management of LUTS/BPE are reported to be approximately $6 billion dollars in the USA [2] . In a population with increasing life expectancy, the economic burden is expected to follow an upward trend in the future [3] .
The treatment of bothersome LUTS/BPE comprises conservative approaches, pharmacological options, and various surgical procedures [4 5] . Medical therapy has a therapeutic ceiling in terms of efficacy and in addition to the associated adverse effects including postural hypotension, dizziness, asthenia, and compromised sexual function are the main reasons for discontinuation.
Transurethral resection of the prostate (TURP) has stood the test of time and is justly considered the surgical reference method offering durable clinical improvement. Although refinements of the technique improved the safety profile of TURP over time, considerable morbidity of 20% and long-term complications including ejaculatory dysfunction (65%), erectile dysfunction (10%), urethral strictures (7%), urinary tract infection (4%), bleeding requiring transfusion (2%), urinary incontinence (2%), and a retreatment rate of 6% have still to be acknowledged [4 5] . The use of laser enucleation of the prostate has emerged as a very effective and potentially more efficacious approach comparable in terms of efficacy to open simple prostatectomy. Laser ablation techniques have also been widely explored in recent years 6 7 8 9 .
The development of novel minimally invasive procedures strives for innovative approaches equally effective to standard techniques with a more favourable safety profile. A true minimally invasive treatment should be cost-effective and easy to perform. It should ensure rapid and durable relief of symptoms and ideally be performed in an ambulatory setting under local anaesthesia. A short recovery time and smooth return to normal activity are important determinants for quality of life after surgery [10 11] . Sexual function including erectile and ejaculatory function is compromised after treatment with current standard techniques, but should be completely preserved by a successful minimally invasive approach unless tissue is ablated. In the past, a plethora of concepts have been abandoned owing to insufficient clinical outcomes or lack of reproducibility.
Novel innovative concepts have been introduced into the interventional spectrum for the management of LUTS and early clinical results seem to be promising. The development of intraprostatic injectables, medical devices, and innovative techniques of tissue ablation have attracted renewed interest in the field. The objective of the current review is to present the early clinical experiences with novel emerging minimally invasive treatment options for male LUTS due to BPE.
Medline, PubMed, the Cochrane database, and Embase were screened for randomised controlled trials (RCTs), clinical trials, and reviews on novel minimally invasive treatment options for male LUTS due to BPE. The authors discussed emerging techniques that were considered novel concepts. Approaches like intraprostatic ethanol injections or prostatic stents have been investigated in the past with modifications over time and therefore these were excluded in this review. As clinical data on new treatment options are scarce the authors decided to provide a narrative review by presenting briefly the basic principles of each technique and the early available clinical data. The objective of this collaborative review is to inform the reader on new advances in the field in an informative and objective way based on published data, without making formal recommendations. We focus on intraprostatic injectables including botulinum neurotoxin A (BoNT/A), NX1207, and PRX302, mechanical devices like the prostatic urethral lift (PUL) and the temporary implantable nitinol device (TIND), new techniques for prostate ablation such as the image guided robotic waterjet ablation (AquaBeam) and procedures based on convective water vapour energy (Rezūm), and finally the prostatic artery embolization (PAE).
Intraprostatic injections for the treatment of prostatic diseases do not represent novel concepts. Early reports of intraprostatic injections for the management of LUTS were documented in 1910 [12] . Novel injectables have been designed to address specifically key processes in the pathophysiology of LUTS due to BPE [13 14] .
The exact mechanisms of BoNT/A still need to be elucidated, but based on current knowledge is likely to involve modulation of sensory neural mechanisms [14] . In the urological field, it has been officially approved for the treatment of idiopathic and neurogenic detrusor overactivity (BOTOX; Allergan, Dublin, Ireland) [15] and has been explored in preliminary work for functional disorders such as detrusor sphincter dyssynergia [16] and bladder pain syndrome/interstitial cystitis [17] . Briefly, it was shown to modulate neurotransmission of sympathic, parasympathic, and sensory nerve terminals in the prostate. The chemo-denervation results in the reduction of size and growth of the prostate and impacts contractility as the dynamic component of benign prostatic obstruction [14] .
Experimental evidence anticipated successful management of LUTS due to BPE; however, results from clinical trials have been falling short of expectations 18 19 20 21 22 . Three RCTs have been conducted to date 23 24 25 . The first small trial by Maria et al [24] enrolled 30 individuals who were randomly assigned to receive 200 U BOTOX or placebo via the transperineal route. One month after treatment the primary endpoints were met with a relevant clinical improvement of 54% as determined by the American Urological Association Symptom Index ( p = 0.00001) and Q max increasing significantly from 8.1 ml/s to 14.9 ml/s ( p = 0.00001). Prostate volume was reduced by 54% ( p = 0.00001) and postvoid residual volume decreased by 60% ( p = 0.00001). Clinical response was stable throughout the follow-up period of 12 mo. No adverse events were reported. This promising initial outcome could not be reproduced in two further larger RCTs. Both phase 2 studies by Marberger et al [23] and McVary et al [25] including 380 and 427 participants, respectively, were not able to demonstrate a relevant benefit of BOTOX over placebo. A recent meta-analysis failed to show the therapeutic efficacy of BoNT/A for a meaningful therapy of male LUTS associated with BPE in clinical practice [26] . Therefore, no recommendation can be made for the intraprostatic injection of BoNT/A for the treatment of male LUTS due to BPE.
This novel cysteine-containing linear protein of proprietary composition with selective proapoptotic features was suggested for the office-based administration via the transrectal route under transrectal ultrasound guidance for the minimally invasive treatment of male LUTS.
After smaller clinical phase 1/2 trials had indicated efficacy and safety of NX-1207 [27 28] , the positive treatment response after intraprostatic application was further confirmed in two larger RCTs, with one demonstrating noninferiority to finasteride 29 30 31 . However, both US phase 3 pivotal studies failed to meet primary endpoints, which prompted the sponsors to prematurely terminate the respective European phase 3 trials. Therefore, NX-1207 failed as an intraprostatic injectable for the treatment of male LUTS.
PRX302 was designed as a first-in-class agent for the therapy of male LUTS due to BPE. The highly toxic pore-forming protein is originally produced as the inactive precursor proaerolysin by the aquatic pathogen Aeromonas hydrophila . Cleavage by furin proteases is necessary for the activation of the precursor to its active form aerolysin, which in turn forms stable pores in the plasma membrane, resulting in cell death. This protein was genetically modified to create a prostate-selective compound. The original cleavage site was replaced with a prostate-specific antigen-specific sequence, creating PRX302. The abundance of active prostate-specific antigen only restricted to the prostate confines the biologic activity of PRX302 exclusively to prostatic tissue 32 33 34 35 36 37 .
Clinical efficacy and safety were assessed and confirmed in a small phase 1 trial on 15 patients and a phase 2 study on 18 patients [38] . PRX302 was injected in the transition zone via the transperineal route under transrectal ultrasound guidance in an office-based setting. In both studies over 60% of treated patients experienced a ≥30% improvement of clinical symptoms compared to baseline as quantified by International Prostate Symptom Score (IPSS) after 360 d. Only the phase 2 trial reported an increase in Q max of ≥3 ml/s in 61% of treated patients out to 12 mo. The impact of PRX302 on prostate volume revealed a reduction of ≥20% in 36% of patients in the phase 1 trial and 63% of participants enrolled in the phase 2 study for the 360-d follow-up. No impairment of erectile function as measured by the International Index of Erectile Function (IIEF) score was observed in either study. Adverse events were mild to moderate and only temporary, resolving within 72 h. A prospective, randomised (2:1), double-blind, vehicle controlled, multicentre phase 2b evaluated 92 patients eligible for inclusion with regard to safety and efficacy of a single transperineal injection [39] . Treatment with PRX302 resulted in a 9-point improvement in IPSS that was stable throughout the follow-up period of 12 mo. Mean change from baseline (PRX302 minus placebo) was 3.3 points after 3 mo ( p = 0.04) and 2.8 points at 12 mo ( p > 0.05). Impact on Q max showed an increase of 3 ml/s that was stable during the study period of 12 mo. No significant effect was observed for the reduction of prostate volume or postvoid residual urine volume. No compromising effect on erectile dysfunction was reported. Adverse events were mild to moderate and transient in nature. Recently, the sponsor (Sophiris Bio Corp, CA, USA) announced that a prospective, randomised, double-blind, placebo-controlled phase 3 trial was successful to meet primary endpoint ( http://investor.sophirisbio.com/releasedetail.cfm?ReleaseID=969508 ). After 12 mo, a reduction in IPSS of 7.6 points after transrectal injection of PRX302 was statistically superior to an improvement of 6.58 points in the vehicle control ( p = 0.043). This encouraging result warrants further randomized controlled trials needed to define the role of the intraprostatic injection of PRX302 in the spectrum of minimally invasive treatment modalities for male LUTS due to BPE.
Mechanical devices to establish and preserve urethral patency have been introduced as temporary or permanent treatment options for bladder outlet obstruction secondary to BPE as an alternative to indwelling catheters or for patients unfit for surgery. Over the years the concept of prostatic stents attracted renewed interest, as technical modifications were constantly developed to optimize relevant issues like a reduction of migration rate, biocompatibility, encrustation, misplacement, and perineal pain after implantation. However, it is the advent of novel mechanical concepts to deobstructing the prostatic lumen that seem to prove their worth as minimally invasive approach.
TIND (Medi-Tate; Medi-Tate Ltd., Or Akiva, Israel) is an emerging device designed to remodel the bladder neck and the prostatic urethra in an unprecedented way ( Fig. 1 ). The TIND is composed of elongated struts and an anchoring leaflet, all made of nitinol. Under cystoscopic guidance the device is preloaded on a dedicated delivery system and advanced through a standard 22-F cystoscope sheath in an up-folded configuration. The dimensions of this tool (total length: 50 mm, outer diameter: 33 mm) were designed to cover the complete prostatic urethra, ranging from bladder neck to proximal to the external urinary sphincter. Under direct visualisation the TIND is deployed inside the bladder in expanded configuration. Anchoring the leaflet slide at the 6 o’clock position distal to the bladder neck ensures precise and safe positioning within the bladder neck and the prostatic urethral lumen. The intended mode of action is to compress obstructive tissue by the expanded device, whose struts will exert radial force leading to ischaemic necrosis in defined areas of interest. The TIND is left in position for 5 d, time enough to create prostatic incisions anteriorly, at the 5 o’clock and 7 o’clock positions. The resulting incisions may be similar to a Turner Warwick incision. In an outpatient setting the device is safely removed by standard urethroscopy.

Fig. 1
Temporary implantable nitinol device (TIND). (A) TIND in its expanded configuration, longitudinal view. (B) TIND in its expanded configuration, front view. (C) Cystoscopic visualisation: the anchoring leaflet is in its correct position (*) and the incisions are visible (---). Length: 50 mm, width: 33 mm.
A single-arm, prospective study on 32 patients was conducted to evaluate feasibility and safety of the procedure [40] . All participants were treated with light sedation, mean operative time was 5.8 min and after the 20th procedure patients were discharged on the same day of intervention. This first study reported that the device was well tolerated by all patients. Overall, four postoperative complications (12.5%) such as prostatic abscess, urinary retention, transient incontinence, and urinary tract infection were recorded. No late complications, adjunctive reinterventions, or medical therapy were documented at the 12-mo follow-up. First functional outcomes suggest efficacy of the technique. After 12 mo, mean changes relative to baseline values were 45% for IPSS and 67% for Q max . This first clinical experience demonstrated that implantation of TIND is a feasible and safe procedure, easy, and fast to perform. Further studies are underway (Clinicaltrials.gov: NCT02145208 ) to evaluate efficacy, durability, and to define patient selection for this innovative approach.
The Urolift procedure (Neotract Inc., Pleasanton, CA, USA) was officially approved by both the Food and Drug Administration and National Institute for Health and Care Excellence within 4 yr after first introduction [41] . The technical goal is to create a continuous anterior channel through the prostatic lumen extending from bladder neck to the verumontanum ( Fig. 2 ) [42] . Tissue retracting implants loaded on a dedicated delivery device are placed anterolaterally at the 2 o’clock and 10 o’clock position under cystoscopic control. On the one hand, preservation of the neurovascular bundles and the dorsal venous plexus is assured. On the other hand, encroaching lateral prostatic lobes are compressed by the implants remodelling an open anterior channel throughout the prostatic fossa. The permanent Urolift implant is composed of a nitinol capsular tab (diameter: 0.6 mm, length: 8 mm), an adjustable polyethylene teraphtalate nonabsorbable monofilament (diameter: 0.4 mm), and a stainless steel urethral end piece (8 mm × 1 mm × 0.5 mm) [41] . Unlike prostatic stents the limited superficial exposure of the urethral end piece minimises the risk of encrustation and after invagination the implants are epithelialized. Usually, the implantation of multiple retractors is required for successful treatment. Patient selection is critical for optimal efficacy of the PUL technique. Strict inclusion criteria were defined for recruitment of patients in large RCTs in order to assess efficacy in a particular subset of LUTS patients. Prostate volumes between 20 cc and 70 cc with typical lateral lobe obstruction ( kissing lobes ) are best addressed. More challenging to treat are prostates larger than 100 cc or patients with a high bladder neck or an obstructing middle lobe and therefore, these anatomical features are considered (relative) contraindications. Notably, after PUL any surgical interventions including TURP or laser-based techniques are still possible without limitation. PUL can be performed under local anaesthesia in an outpatient setting and in most cases no postinterventional catheterisation is required.

Fig. 2
Prostatic urethral lift: Urolift. (A) Retracting implant composed of a nitinol capsular tab, a polyethylene terephthalate monofilament and a stainless steel urethral end piece. (B) Benign prostatic obstruction by encroaching lateral lobes. (C) Delivery of retracting implant. (D) Expanded urethra after compression of lateral lobes.
Efficacy and safety were confirmed in the multicentre, prospective, randomised, controlled, and blinded L.I.F.T study enrolling 206 patients who were randomized 2:1 between PUL and sham [43] . The primary end point at 3 mo was met with a 50% reduction in American Urological Association Symptom Index from baseline 22.1 points to 11 points ( p < 0.0001). This improvement was 88% greater than the change in the sham control group and was stable during the study period of 12 mo. At 3 mo, Q max increased significantly by 64% from 8.1 ml/s to 12.4 ml/s ( p < 0.0001) in the PUL group and remained stable up to 12 mo. The therapeutic effect on voiding parameters like Q max was more pronounced than in the control arm ( p < 0.005). So was the case with changes for quality of life assessment and additional outcome measure like the Benign Prostatic Hyperplasia Impact Index (BPHII). No relevant influence of PUL was observed for postvoid residual urine volume. Safety profile was favourable with adverse events reported to be mild to moderate and resolved within 2 wk. Evaluation of sexual function utilising instruments like the IIEF score, and the Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire demonstrated no compromising impact of PUL on erectile and ejaculatory function. With regard to durability it is important to highlight that the rapid clinical response after PUL was stable in follow-up evaluations up to 5 yr. Recently, at the annual meeting of the European Association of Urology 2017 the follow-up data of the L.I.F.T study was presented [44] . The initial improvements in IPSS and Quality of Life (QoL) 1 mo after PUL were 44% and 42% ( p < 0.001), respectively, and changes remained improved at the 5-yr assessment with 38% for IPSS and 54% for QoL ( p < 0.001). The increase in Q max was still 41% at 5 yr. Sexual function was preserved through 5 yr. In 2015, data of a prospective, randomised, controlled trial at 10 European centres on 80 men with LUTS due to BPE was published comparing clinical outcomes after PUL with the reference method TURP [45] . A novel objective outcome tool termed BPH6 was introduced, whose clinical significance has not been evaluated yet. It is composed of the following six domains to evaluate various aspects of efficacy and safety: relief from LUTS, recovery experience, erectile function, ejaculatory function, continence preservation, and safety. Significant amelioration of LUTS was achieved for both procedures. Impact on IPSS, Q max , and postvoid residual urine volume was considerably stronger after TURP ( p < 0.05), whereas PUL was superior to TURP in terms of quality of recovery ( p = 0.008) and preservation of ejaculatory function metrics ( p < 0.0001). No relevant difference was reported for erectile function, incontinence, and safety. Reinterventions due to insufficient treatment response over the study period of 12 mo were necessary in 6.8% and 5.7% of patients after PUL and TURP, respectively. The 2-yr results of the BPH6 study confirmed superiority of TURP over PUL regarding IPSS and Q max , whereas PUL showed sustainable benefit over TURP for quality of recovery and ejaculatory function [46] . Throughout the 2-yr follow-up six patients in the PUL arm (13.6%) and two patients treated with TURP (5.7%) underwent secondary intervention due to return of LUTS. Additional clinical trials and meta-analysis of good quality substantiate that PUL indeed is a minimally invasive procedure able to provide rapid and durable relief of LUTS with acceptable re-intervention rates without compromising sexual function [42 43 45 46 47 48 49 50 51 52 53 54 55 . As a consequence, PUL has been introduced in the spectrum of surgical treatment options for the management of male LUTS due to BPE by current guidelines [4] . Nevertheless, further studies are warranted to evaluate long-term efficacy and safety and to define the position of PUL among various treatment modalities.
AquaBeam (Procept BioRobotics, Redwood Shores, CA, USA) represents a novel and innovative high-end technology in the field of minimally invasive treatment options for LUTS due to BPE. It uses the principle of hydrodissection to effectively ablate prostatic parenchyma while sparing collagenous structures like blood vessels and the surgical capsule. A targeted high velocity saline stream ablates prostatic tissue without the generation of thermal energy, which in turn minimises the risk of thermal injury or collateral tissue damage. Combining waterjet ablation with the obvious benefits of real-time transrectal ultrasound guidance and the accuracy of robotic assistance makes AquaBeam one of the most engineered and targeted approaches for selective ablation of the prostate. The system is composed of three main components: the console/pump, the robotic-controlled hand-piece, and a transrectal ultrasound probe ( Fig. 3 ). The hand-piece is advanced through a 22-F cystoscope sheath until the device is located within the bladder. Next, the hand-piece is locked in place and registered within the prostate and using a biplane transrectal ultrasound probe. Now the surgeon is able to complete the surgical mapping on a touch-screen by defining the spatial dimensions for ablation using the transrectal ultrasound images. This also enables preservation of key anatomical landmarks relevant for both urinary continence and antegrade ejaculation [56] . Patient information is entered manually into the console and then using foot pedal activation aquablation is commenced with the ablation being completely automated. Variation of the high velocity saline flow rate regulates the depth of penetration while longitudinal and rotational movement of the hand-piece follow the predefined dimensions of tissue to be removed. After completion of ablation haemostasis is performed with a Foley balloon catheter on light traction or diathermy or low-powered laser if necessary [57] .

Fig. 3
Aquablation – image guided robotic waterjet ablation: AquaBeam. (A) The Aquablation system is composed of three main components: the console, the robotic hand-piece, and a transrectal ultrasound probe. (B) Tip of the Aquablation probe. The terminal balloon is retracted for sealing of the bladder neck. The trough enables Aquablation. (C) Graphic control interface.
In a prospective, nonrandomised, single-centre trial including 15 men with moderate-to-severe LUTS the feasibility and safety of AquaBeam were confirmed [58] . One-year results of a prospective single-arm multicentre phase 2 trial on 21 men supported safety and efficacy of aquablation [59] . Duration of the procedure averaged 38 min and mean aquablation treatment time was 5 min. In 20 patients, catheters were removed within the 1st 24 h of the procedure and most patients were discharged 1 d after the procedure. No cases of urinary incontinence, erectile dysfunction, or retrograde ejaculation were reported. After 12 mo, IPSS was reduced from 23.0 points at baseline to 6.8 points ( p < 0.001). An increase from 8.7 ml/s to 18.3 ml/s in Q max was demonstrated ( p < 0.0001). At 12 mo the average prostate size showed a reduction from 57 cc to 35 cc ( p < 0.001). This first clinical experience provided encouraging results, but further modifications of the AquaBeam system will be necessary. Anatomical prostatic features like a prostate volume >100 cc and the presence of a large middle lobe are currently limitations to the technology and therefore considered relative contraindications. Further RCTs are underway to evaluate efficacy, durability, and safety of this innovative approach.
Ablation using the Rezūm system (NxThera, Inc., MapleGrove, MN, USA) takes advantage of the thermodynamic principle of convective energy transfer, which is in contrast to conductive heat transfer techniques as applied by known minimally invasive treatment options like transurethral microwave therapy or transurethral needle ablation. In this case, radiofrequency power is used to create thermal energy in the form of water vapor, which in turn deposits the stored thermal energy when the steam phase shifts to liquid upon cell contact. The Rezūm system is composed of a generator containing a radiofrequency power supply to create water vapor from sterile water and a single-use transurethral delivery device. The tip of the delivery device contains an 18-gauge polyether ether ketone needle where 12 small emitter holes spaced circumferentially at 120° intervals allow for targeted dispersion of water vapor into the tissue. The injection is performed at approximately 103° and exceeds slightly interstitial pressure. Due to the convective properties of water vapor the steam disperses rapidly and homogenously through the tissue interstices and releases stored thermal energy onto prostatic tissue effecting cell necrosis. Of note, when the transition zone is treated, energy deposition is contained within the zonal anatomy of the prostate. Histologic and imaging studies using Gadolinium-enhanced magnetic resonance imaging after treatment provided evidence that coalesced thermal lesions were limited to the transition zone without extension to the peripheral zone, bladder, rectum or striated urinary sphincter [60 61] . It was shown that 6 mo after treatment the total prostate volume was reduced by 28.9% and the resolution of thermal lesions as determined with Gadolinium-enhanced magnetic resonance imaging was almost complete [61] . The procedure can be performed in an office based setting with minimal pain management. Under cystoscopic guidance, the needle is positioned at 90° to the area of interest and a 9-s injection of water vapor is achieved. Usually, one to three injections are needed for each lateral lobe and one to two injections may be delivered into the median lobe. Of course, the total number of injections may vary according to prostate size and length of the urethra.
Clinical 1-yr outcomes of men with LUTS due to BPE treated in smaller pilot trials provided first evidence for efficacy and safety of the Rezūm system [62] . In the first multicentre, randomised, controlled study 197 men were enrolled and randomised in a 2:1 ratio to treatment with the Rezūm system or control [63] . The mock procedure was rigid cystoscopy with imitated treatment sounds. The primary efficacy end point was met at 3 mo with relief of symptoms measured by a change in IPSS of 50% for the treatment arm compared to 20% for the control group ( p < 0.0001). In the thermal treatment arm, Q max increased significantly by 67% from 9.9 ml/s to 16.1 ml/s ( p < 0.0001) after 3 mo and this positive clinical outcome was sustained throughout the study period with an improvement of 54% at the 12-mo follow up. No relevant impact was observed on postvoid residual urine volume. Outcome for QoL was significantly improved 2 wk after intervention and followed a positive trend over the study period with a meaningful treatment response of 51% at 12 mo ( p < 0.0001). Further validated objective outcome measures such as BPHII, Overactive Bladder Questionnaire Short Form for overactive bladder bother, and impact on QoL and International Continence Society Male Item Short Form Survey for male incontinence demonstrated significant amelioration of symptoms at the 3-mo follow-up with sustained efficacy throughout the study period of 12 mo. Direct comparison of determined end points at the 3-mo follow-up including IPSS, Q max , BPHII, Overactive Bladder Questionnaire Short Form proved statistically significant superiority of the thermal therapy over the sham procedure. Safety profile was favourable with adverse events documented to be mild to moderate and resolving rapidly. Of note, almost 69% received only oral sedation and in contrast to most of the novel minimally invasive techniques all critical prostatic zones including the middle lobe were successful treated. Preservation of erectile and ejaculatory function after convective water vapor thermal therapy was demonstrated utilizing validated outcome instruments such as IIEF and Male Sexual Health Questionnaire-Ejaculation Disorder Questionnaire [64] . The recently reported 2-yr results confirmed durability of the positive clinical outcome after convective water vapour energy ablation [65] . The first clinical experience suggests that this novel technique of prostatic ablation is able to provide rapid and meaningful relief of LUTS without compromising sexual function. Further RCTs are needed to confirm these promising first clinical results and to evaluate mid-term and long-term efficacy and safety of the Rezūm system.
One of the first nominal case reports of prostatic artery embolization (PAE) was a necessary therapeutic intervention with an unexpected positive side effect. A 76-yr-old patient with relevant cardiac comorbidities precluding standard surgical intervention and a complicated course of BPE refractory to pharmacotherapy developed recurrent episodes of acute urinary retention and persistent gross haematuria [66] . After unilateral super-selective transarterial prostate embolization colleagues were not only successful in managing prostatic bleeding instantly, but observed also some treatment effect on his male LUTS/BPE. The obvious advantage of PAE is that it can be performed as a 1-d case under local anaesthesia for the access through the femoral arteries. Digital subtraction angiography displays arterial anatomy and the appropriate prostatic arterial supply is selectively embolized using nonspherical polyvinyl alcohol to effect stasis in treated prostatic vessels. Among centres a debate is still ongoing considering the efficacy of a unilateral approach and the benefit of a bilateral approach [67 68] . At the moment, PAE is exclusively performed by interventional radiologists at specialised centres. Atherosclerosis, excessive tortuosity of the arterial supply and the presence of adverse collaterals are anatomical obstacles for the technical approach. Nontargeted embolization may therefore lead to ischaemic complications like transient ischaemic proctitis, bladder ischaemia, or seminal vesicles ischaemia 69 70 71 . Short-term complications including urethral burning sensation, nausea and vomiting are common and have been coined the “post-PAE syndrome” [72] . The extended duration of the procedure with the requirement of fluoroscopy brings the risk of a relevant radiation exposure, which may result in skin irritation, and even burns [73] . But constantly modified low-dose protocols and pulsed frame rate digital subtraction angiography appear to reduce efficiently radiation dosage. Furthermore, contrast toxicity with the need for angiography is another adverse effect that needs to be acknowledged.
In 2013 the prospective nonrandomised trial by Pisco et al [71] evaluated PAE in 255 patients for the treatment of moderate to severe LUTS due to BPE at a single centre. The technical procedure, defined as either unilateral or bilateral embolization, was technically successful in 97.9% and 88% of treated patients were discharged on the same day of intervention. In this study, the mean procedure time was 73 min and the mean fluoroscopy time was 18 min, which is among the shortest fluoroscopy times mentioned in the literature and should therefore not be considered representative for the procedure. Clinical outcome parameters demonstrated efficacy of PAE. At the 3-mo follow-up a significant impact on LUTS as measured by IPSS with a mean reduction from 24 to 11 points and an improvement in QoL with a mean change from 4.4 to 2.23 points were reported (both p < 0.0001). Q max increased from 9.2 ml/s to 12.4 ml/s and postvoid residual urine volume decreased from 102.9 ml to 59.2 ml (both p < 0.0001). Mean prostate volume changed from 83.5 cc to 68.3 cc at 3 mo. The positive treatment responses were stable up to 12 mo. Neither cases of retrograde ejaculation were recorded, nor impairment of erectile function as determined using the IIEF questionnaire was documented. Similar results confirming technical success and suggesting efficacy without compromising sexual function were obtained in additional uncontrolled pilot studies [68 74 75 76 77 78 79 80 81 82 . Two prospective RCTs were conducted for direct comparison of PAE with the reference method TURP [83 84] . Both studies observed significant treatment outcomes for both procedures as compared to baseline values, but TURP was always superior considering urodynamic parameters such as Q max and postvoid residual urine volume. Improvement of LUTS as determined by IPSS and QoL was more pronounced after TURP and reduction of prostate volume was significantly more efficient after TURP than PAE. Another 1-yr matched-pair analysis compared PAE to open prostatectomy for management of LUTS due to BPE and reported significantly superior functional outcomes as determined by IPSS, QoL, Q max , and postvoid residual urine volume for open prostatectomy [85] . Altogether, available data indicate a high technical success rate and suggest some clinical benefit for the treatment LUTS.
However, a recently published systematic review with meta-analysis and meta-regression on available data concluded that PAE should still be considered an experimental approach [86] . RCTs of good quality are still missing to justify this interesting technique on an elective indication and are currently ongoing. The selection of LUTS patients who will benefit from PAE still need to be defined. It is important to stress, that all of the presented novel minimally invasive treatment modalities above are able to specifically target the critical areas of bladder outlet obstruction secondary to BPE. In contrast, PAE impacts the entire prostate without the option for focused and controlled action on bladder outlet obstruction. This may explain the higher clinical failure rate compared to reference methods like TURP and commonly observed complications like acute urinary retention in almost 26% of cases [84] . Management of LUTS due to BPE must be handled by urologists. A multidisciplinary team approach of urologists and radiologists is mandatory as the basis for future RCTs of good quality in order to integrate this promising option in the spectrum of efficient minimally invasive treatment options.
Many novel and innovative techniques have arrived with the main objective to establish effective strategies for the relief of male LUTS with a more favourable safety profile. Intraprostatic injectables have fallen short of expectations in clinical trials. PRX302 is the only substance that showed safety and efficacy in a phase 3 trial. Mechanical devices like the PUL procedure are supported by evidence of good quality and it was clearly demonstrated that it provides rapid and long-term relief of LUTS without compromising sexual function. TIND has recently been introduced with promising functional outcomes, but further RCTs are warranted to fully evaluate its potential in the field of minimally invasive therapies. New ablative approaches like the image guided robotic waterjet ablation (AquaBeam) or procedures based on convective water vapour energy (Rezūm) are currently under evaluation. Further trials are needed to demonstrate their therapeutic potential and advantages compared to standard techniques. With regard to PAE, a substantial high clinical failure rate and a specific spectrum of complications not common after urologic interventions are of concern. A multidisciplinary approach with both urologists and radiologist is necessary to define its role as a potential option among the established treatment modalities. A synopsis of the main characteristics of the emerging treatment modalities is depicted in Table 1 . Fundamental research in the development of novel techniques and their clinical assessment have progressed substantially, and will show which approach will pass the test of time.
| Minimally invasive treatment | Feasability | Safety | Efficacy | Durability | Preservation of sexual function | Approval | (Relative) contraindication | ||
|---|---|---|---|---|---|---|---|---|---|
| Short-term (1 yr) | Mid-term (3 yr) | Long-term (5 yr) | |||||||
| Intraprostatic injection | |||||||||
| BoNT/A | + | + | – | ||||||
| NX1207 | + | + | – | ||||||
| PRX302 | + | + | + | + | NA | NA | + | No | No |
| Mechanical devices | |||||||||
| TIND | + | + | + | + | NA | NA | NA | No | Middle lobe |
| Urolift | + | + | + | + | + | + | + | FDA, NICE | PV > 100 cc, middle lobe |
| Ablative techniques | |||||||||
| AquaBeam | + | + | + | NA | NA | NA | + | No | PV > 100 cc, middle lobe |
| Rezũm | + | + | + | + | NA | NA | + | No | No |
| Prostatic artery embolization | + | + | + | + | NA | NA | NA | No | No |
Table 1Synopsis of main characteristics of emerging minimally invasive treatment options for male lower urinary tract symtoms (contraindications refer to specific prostatic configurations; sexual function includes both erectile and ejaculatory function)
Author contributions: Giuseppe Magistro had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Magistro, Gratzke.
Acquisition of data: Magistro, Gratzke.
Analysis and interpretation of data: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Drafting of the manuscript: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Critical revision of the manuscript for important intellectual content: Magistro, Chapple, Elhilali, Gilling, Roehrborn, McVary, Stief, Woo, Gratzke.
Statistical analysis: None.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Magistro, Chapple, Gratzke.
Other: None.
Financial disclosures: Giuseppe Magistro certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Chapple reports personal fees and nonfinancial support from Allergan, grants, personal fees, and nonfinancial support from Astellas, personal fees and nonfinancial support from Boston, personal fees and nonfinancial support from Medtronic, personal fees from Pfizer, personal fees and nonfinancial support from Recordati, outside the submitted work. Gilling reports activity as study investigator and meeting participant for PROCEPT BioRobotics Inc. McVary is a consultant/investigator for Allergan, Lilly/ICOS, NxThera, NIDDK, Astellas, Boston Scientific, and Sophiris. Woo reports personal fees from Boston Scientific, other from NxThera, other from Neotract, other from GSK, outside the submitted work. Gratzke reports grants/honoraria/ consultation fees from Astellas, Bayer, GSK, MSD, Recordati, Amgen, Ipsen, Janssen, Lilly Pharma, Recordati, Pfizer, Rottapharm, and STEBA.
Funding/Support and role of the sponsor: None.