Background
Robot-assisted simple prostatectomy (RASP) is a minimally invasive procedure for treatment of patients with lower urinary tract symptoms (LUTS) due to large benign prostatic enlargement (BPE).
Objective
To present the perioperative and short-term functional outcomes of RASP in a large series of patients with LUTS due to BPE treated in a high-volume referral center.
Design, setting, and participants
We retrospectively collected data for 67 consecutive patients who underwent RASP from October 2008 to August 2014.
Surgical procedure
RASP was performed using a Da Vinci S or Si system with a transvesical approach.
Measurements
Complications were graded according to the Clavien-Dindo system. Continuous variables are reported as median and interquartile range (IQR). Comparison of preoperative and postoperative outcomes was assessed by Wilcoxon test. A two-sided value ofp < 0.05 was considered statistically significant.
Results and limitations
The median preoperative prostate volume was 129 ml (IQR 104–180). For the 45 patients who did not have an indwelling catheter, the median preoperative International Prostate Symptom Score (IPSS) was 25 (20.5–28), the median maximum flow rate (Qmax) was 7 ml/s (IQR 5–11), and the median post-void residual volume (PVRV) was 73 ml (IQR 40–116). The median operative time was 97 min (IQR 80–127) and the median estimated blood loss was 200 ml (IQR 115–360). The postoperative complication rate was 30%, including three cases (4.5%) with grade 3b complications (major bleeding requiring cystoscopy and coagulation). The median catheterization time was 3 d (IQR 2–4) and the median length of stay was 4 d (IQR 3–5). The median follow-up was 6 mo (IQR 2–12). At follow-up, the median IPSS was 3 (IQR 0–8), the medianQmaxwas 23 ml/s (IQR 16–35), and the median PVRV was 0 ml (IQR 0–36) (allp < 0.001 vs baseline values). The retrospective design is the major study limitation.
Conclusions
Our data indicate good perioperative outcomes, an acceptable risk profile, and excellent improvements in patient symptoms and flow scores at short-term follow-up following RASP.
Patient summary
We analyzed the perioperative and functional outcomes of robot-assisted simple prostatectomy in the treatment of male patients with lower urinary tract symptoms due to large prostatic adenoma. The procedure was associated with a relatively low risk of complications and excellent functional outcomes, including considerable improvements in symptoms and flow performance. We can conclude that the procedure is a valuable option in the treatment of such patients. However, comparative studies evaluating the efficacy of the procedure in comparison with endoscopic treatment of large prostatic adenomas are needed.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).
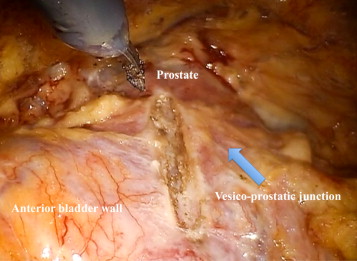
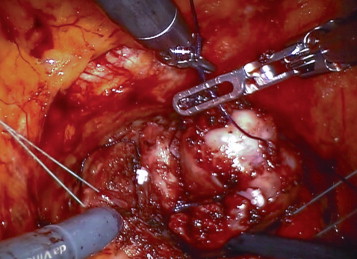
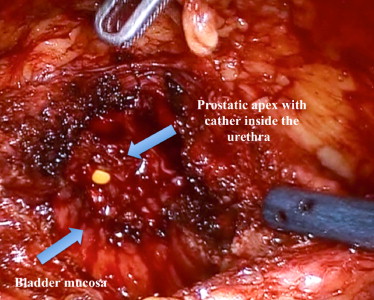
The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.
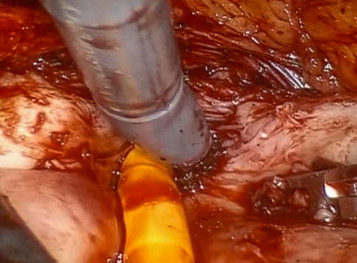
Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).
A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
The surgical management of large benign prostatic hyperplasia remains a challenge. Methods have evolved over the last 30 yr from the traditional open retropubic or transvesical simple prostatectomy, to transurethral techniques including transurethral resection of the prostate (TURP), laser enucleation (holmium:YAG laser enucleation of the prostate, HOLEP), and vaporization methods, as well as laparoscopic simple prostatectomy [1] . Open prostatectomy (OP) and HOLEP currently represent the mainstay of surgical management for large adenomas, particularly for glands larger than 100 g in size, and these techniques have the largest evidence base in the literature [2] . However, OP remains a challenging procedure with a significant complication rate, and HOLEP, although associated with very good postoperative outcomes and low overall complications rates, is still not easy to adopt owing to a perceived steep learning curve and equipment costs[3], [4], [5], and [6].
To adopt the benefit of minimally invasive surgery for OP, laparoscopic simple prostatectomy and, more recently, robot-assisted simple prostatectomy (RASP) have been developed. Specifically, with the widespread adoption of robotic surgery for prostate cancer, urologists have become very accustomed to operating on the prostate in a laparoscopic environment, allowing a natural transition to simple prostatectomy for benign gland enlargement. A number of centers have reported small case series with perioperative and functional outcomes similar to HOLEP and OP[7], [8], [9], [10], [11], [12], and [13].
Here we present our surgical technique for RASP and perioperative and short-term functional outcomes in a large series of patients with LUTS due to benign prostatic enlargement treated in a high-volume referral center for robotic surgery.
Ethics review board approval was waived for this retrospective review of patient files. Between April 2008 and October 2014, 67 consecutive men underwent RASP in our institution and were included in this analysis. All patients were offered initial medical management where appropriate. Patients failing medical management or those requiring immediate surgery with a prostate gland larger than 100 g were offered RASP. Patients were counseled on the risks and benefits of the procedure, and were listed for surgery after providing informed consent. All patients underwent initial clinical work-up including history, physical examination, flow rate evaluation including peak flow rate (Qmax) measurement, voided volume, and residual volume measurement via transabdominal ultrasound; renal tract ultrasound, transrectal ultrasound (TRUS) prostate volume measurement, prostate-specific antigen (PSA) testing and International Prostate Symptom Score (IPSS) assessment. Postoperative follow-up occurred at 6 wk, and then annually either at our center or with the referring urologist. Postoperative assessment included physical examination, flow rate assessment, residual urine measurement, renal tract ultrasound, PSA, and IPSS.
All procedures were performed by one of two surgeons using a Da Vinci Si surgical system (Intuitive Surgical, Sunnyvale, CA, USA) in the four-arm configuration via a transperitoneal approach.
After induction of general anesthesia, the patient is placed in the lithotomy position at a steep Trendelenburg angle with padding of pressure points. The patient receives a single perioperative dose of antibiotic prophylaxis. Our technique uses placement of five trocars similar to that for radical prostatectomy, including a 12-mm camera trocar placed supraumbilically; two 8-mm robotic trocars bilateral on a line between the camera port and the iliac crest at 8 cm from the camera port; and another 8-mm robotic trocar on the left side at 8 cm from the other robotic port and at the same level as the camera port. A lateral 12-mm port is placed 2 cm cranial of the iliac crest on the right side for the assistant ( Fig. 1 ). If needed, an additional 5-mm trocar is placed in between the camera port and the right robotic port for a suction device. Three instruments are used: Hot Shears (Intuitive Surgical) monopolar curved scissors, ProGrasp forceps (Intuitive Surgical), and a large needle driver.

The bladder is filled with 100 ml of saline via an indwelling catheter and then released from the anterior abdominal wall. After bladder dropping, a vertical cystotomy is made starting just above the prostate-vesical junction ( Fig. 2 ).

The position of the ureteric orifices is determined to ensure safety during resection, and an initial incision is made at the edge of the adenoma between the 12- and 2-o’clock positions to find the correct plane between the adenoma and the peripheral zone of the gland. This plane is developed bluntly and sharply circumferentially on both sides of the prostate. Vicryl 1-0 stay stitches are used to provide traction on the adenoma to assist dissection. Vicryl stay sutures can also be used where necessary to evert the bladder edges to improve visualization ( Fig. 3 ). The dissection is carried out as far distally as possible without risking injury to the sphincteric complex. At this point an anterior commissurotomy is made, incising with diathermy onto the urethral catheter, allowing the apex of the adenoma to be freed via precise, safe dissection ( Fig. 4 ). The adenoma is collected in an Endo Catch bag (Covidien, Mansfield, MA, USA) and placed in the right or left iliac fossa.


Bleeding vessels in the prostatic bed are oversewn with a 3-0 monocryl suture. The prostatic fossa is then retrigonized by advancing the bladder neck mucosa as far distally to the prostate apex as possible using a double-layer 3-0 V-Loc (Covidien) running suture, taking care to avoid incorporation of the ureteric orifices ( Fig. 5 ).

A 20F three-way irrigation catheter is placed and the cystotomy is closed in two layers using 3-0 V-Loc. Bladder irrigation with normal saline is continued overnight. To prevent venous thromboembolism (VTE), patients wear TED compression stockings (Covidien) during hospitalization and receive subcutaneous low–molecular-weight (LMW) heparin injections from the first postoperative night. The bladder catheter is typically removed on postoperative day 1–3, depending on the clarity of urine drainage. Patients are discharged with 10 d of nitrofurantoin for antibiotic prophlyaxis and 21 d of subcutaneous LMW heparin injections for VTE prophylaxis.
Retrospective data collected included the following patient characteristics: patient age; Charlson comorbidity index; pre- and postoperative PSA, IPSS and uroflowmetry; prostate size on TRUS; and preoperative catheterization. Perioperative data collected included operative time, estimated blood loss, intra- and postoperative complications, pre- and postoperative hemoglobin, blood transfusion, catheterization duration, and length of stay. Complications were graded according to the Clavien-Dindo classification [14] . Finally, pathologic records were reviewed to obtain specimen weights and any nonbenign histology findings.
Continuous variables are reported as median and interquartile range (IQR). Comparisons of preoperative and postoperative outcomes were assessed using the Wilcoxon test. Univariate and multivariate regression models were used to assess predictors of any grade complications. A two-sided value ofp < 0.05 was considered statistically significant. All statistical tests were performed with SPSS version 22.0 (SPSS, Chicago, IL, USA).
Table 1 lists data for baseline patient characteristics and perioperative outcomes. Seven patients underwent concomitant removal of bladder stones; a single patient underwent simultaneous excision of a bladder diverticulum. Notably, no intraoperative complications occurred.
Table 1 Baseline patient characteristics and perioperative outcomes
| Parameter | Value |
|---|---|
| Patient demographics | |
| Age (yr) | 69 (66–75) |
| Charlson comorbidity index | 2 (0–3) |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) |
| Prostate volume (ml) | 129 (104–180) |
| International Prostate Symptom Score | 25 (20.5–28) |
| Maximum flow rate (ml/s) | 7 (5–11) |
| Voided volume (ml) | 121 (80–190) |
| Post-void residual volume (ml) | 73 (40–116) |
| Suprapubic catheter before surgery | 19 (29) |
| Indwelling urethral catheter before surgery | 4 (6) |
| Perioperative parameters | |
| Operative time (min) | 97 (80–127) |
| Estimated intraoperative blood loss (ml) | 200 (115–360) |
| Postoperative transfusion | 1 (1.5) |
| Catheterization duration (d) | 3 (2–4) |
| Length of hospital stay (d) | 4 (3–5) |
| Preoperative hemoglobin (g/dl) | 14.3 (13.7–15.2) |
| Postoperative hemoglobin (g/dl) | 13.2 (11.7–14.1) |
| Specimen weight (g) | 84 (65–114) |
Data are presented as median (interquartile range) for continuous variables and asn(%) for categorical variables.
Table 2 lists the complications observed within 30 d for our cohort. A total of 23 complications were observed in 20 patients (30%). According to the highest grade they experienced, ten patients (15%) experienced grade 1, four (6%) experienced grade 2, three (4.5%) experienced grade 3a, and three (4.5%) experienced grade 3b complications. No grade 4 or 5 complications occurred. The three grade 3a complications involved patients requiring flexible cystoscopy for recatheterization (n = 1), removal of a small bladder stone (n = 1), and confirmatory cystoscopy for severe storage symptoms at the first follow-up (n = 1). The three grade 3b complications required a return to theatre for cystocoagulation to control bleeding within the first 48 h. All three cases occurred in the initial phase of the surgeons’ learning curves.
Table 2 Perioperative complications stratified by grade according to the Clavien-Dindo classification (23 complications observed in 20 patients)
| Postoperative complication | n (%) |
|---|---|
| Grade 1 | |
| - Constipation | 4 (6) |
| - Wound bleeding/infection | 5 (7.5) |
| - Hypokalemia | 1 (1.5) |
| - Vasovagal syncope | 1 (1.5) |
| - Subcutaneous intravenous infusion leak | 1 (1.5) |
| Grade 2 | |
| - Blood transfusions | 1 (1.5) |
| - Urinary tract infections | 3 (4.5) |
| - Pneumonia | 1 (1.5) |
| Grade 3a | |
| - Cystoscopy for recatheterization | 1 (1.5) |
| - Cystoscopy for removal of a small bladder stone | 1 (1.5) |
| - Cystoscopy for severe storage at first follow-up visit | 1 (1.5) |
| Grade 3b | |
| - Cystoscopy for gross hematuria | 3 (4.5) |
| Complications by highest grade experienced by each patient | |
| - Grade 0 | 47 (70) |
| - Grade 1 | 10 (15) |
| - Grade 2 | 4 (6) |
| - Grade 3a | 3 (4.5) |
| - Grade 3b | 3 (4.5) |
| - Grade 4 | 0 |
| - Grade 5 | 0 |
Constipation was defined as an inability to achieve a bowel movement by postoperative day 3 with no signs of ileus or small bowel obstruction. Hypokalemia was defined as a blood potassium level of <3.5 mmol/l. A syncopal event was defined as a brief loss of consciousness due to drop in heart rate and blood pressure.
Table 3 summarizes predictors of complications of any grade. Age (odds ratio [OR] 1.6;p = 0.028) and number of procedures (OR 0.9;p = 0.01) were independent predictors of complications of any grade.
Table 3 Univariate and multivariate analyses of predictors of any grade of complication among 67 patients who underwent robot-assisted simple prostatectomy
| Parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 0.9 (0.9–1) | 0.002 | 1.6 (1.1–2.4) | 0.028 |
| Charlson comorbidity index | 0.6 (0.5–0.8) | 0.001 | 0.9 (0.5–1.8) | 0.817 |
| Prostate volume | 0.9 (0.8–1) | 0.004 | 1.0 (1.0–1.1) | 0.953 |
| Ordinal number of procedures | 1.0 (0.9–1.0) | 0.017 | 0.9 (0.9–1) | 0.01 |
OR = odds ratio; CI = confidence interval.
On pathologic examination, the median specimen weight for excised adenomas was 84 g (IQR 65–114). Ten men (15%) were found to have small-volume prostate cancer. Nine of these were Gleason 3 + 3, while one patient (age 77 yr) had small-volume Gleason 4 + 3 cancer. This patient was managed with surveillance; a recent measurement revealed PSA of 0.19 ng/ml.
The median follow-up was 6.0 mo (range 2–12 mo). Two patients aged 79 and 82 yr at surgery have died from unrelated causes since their surgery. Five other patients were originally from overseas and no follow-up data were available. Table 4 summarizes the functional results at follow-up. Patients showed significant improvements in baseline IPSS and flow rate parameters. Median IPSS improved from 25 preoperatively to 3 postoperatively (p < 0.001). The medianQmaximproved from 7 ml/s preoperatively to 23 ml/s postoperatively (p < 0.001). The median post-void residual volume improved from 73 ml preoperatively to 0 ml postoperatively (p < 0.001). No patient reported stress urinary incontinence at catheter removal or follow-up evaluation.
Table 4 Preoperative and postoperative IPSS, uroflowmetry parameters, and PSA values
| Variable | Preoperative | Postoperative | p value |
|---|---|---|---|
| International Prostate Symptom Score | 25 (20.5–28) | 3 (0–8) | <0.001 |
| Maximum flow rate (ml/s) | 7 (5–11) | 23 (16–35) | <0.001 |
| Voided volume (ml) | 121 (80–190) | 152 (61–265) | 0.054 |
| Post-void residual volume (ml) | 73 (40–116) | 0 (0–36) | <0.001 |
| Prostate-specific antigen (ng/ml) | 6.5 (3.8–12) | 0.6 (0–1.3) | <0.001 |
Results are presented as median (interquartile range).
Here we report our initial experience with RASP in the treatment of patients with LUTS due to large prostatic adenoma. We observed excellent short-term functional outcomes, with considerable improvements in IPSS andQmaxand a major decrease in PSA, suggesting the presence of a very limited amount of prostatic tissue after surgery. The procedure appears to be relatively safe because of the short operative time and limited blood loss. According to our findings, the risk of postoperative complications is not minimal, but complications probably depend on the nature of the prostate anatomy rather than on the robotic approach. However, most of the complications observed were of low grade and the number of high-grade complications was relatively low, with no grade 4 or 5 complications.
European Association of Urology guidelines recommend OP and HOLEP as the first-choice surgical treatment in men with prostate size >80 ml needing surgical treatment [1] . Specifically, OP can guarantee excellent functional results, including significant improvements in patient symptoms and uroflowmetry parameters[15], [16], [17], and [18]. However, the procedure is associated with a significant risk of complications, including blood loss, blood transfusions (7–8%), and reoperation (1–4%)[15], [17], and [18]. Conversely, HOLEP represents a minimally invasive endoscopic treatment that can provide excellent short- and intermediate-term results in comparison with other endoscopic treatments such as monopolar TURP [2] . A recent meta-analysis of the very few randomized controlled trials comparing HOLEP and OP in glands larger than 100 g demonstrated that HOLEP in this setting was associated with longer operative time (mean difference 24.9 min;p = 0.01) but lower risk of blood transfusions (relative risk 6.09;p < 0.0001), shorter catheterization duration (mean difference 98 h;p = 0.01), and shorter hospital stay (mean difference 4.3 d;p = 0.004) compared with OP. Functional results were similar for the two procedures [2] .
Following the widespread adoption of laparoscopic and, more commonly, robotic surgery for treatment of prostate cancer and other urologic disease, a number of centers have recently reported RASP series demonstrating favorable perioperative and functional outcomes[7], [8], [9], [10], [11], [12], and [13]. However, most these series were very small (patient numbers ranging from 6 to 35) and results were presented for short follow-up. Autorino et al [19] recently reported data for a very large multi-institutional collaboration covering 1300 patients who underwent either laparoscopic or robotic simple prostatectomy.
To the best of our knowledge, our study represents the largest RASP series reported to date. Our excellent functional outcomes lend weight to the current literature and support RASP as a very effective treatment option for large BPH. Our RASP technique is similar to open transvesical simple prostatectomy but differs in some minor aspects from the description of RASP as performed by Leslie et al [7] . Specifically, we approach the adenoma through an incision in the anterior bladder wall and the proximal part of the prostatic capsule, after dropping the bladder from the anterior abdominal wall. By contrast, Leslie et al [7] performed an incision in the bladder dome without detaching the prostate from the abdominal wall. Dissection of the adenoma is performed in a similar way in both techniques, although we start our dissection anteriorly, whereas Leslie et al started with incision of the bladder mucosa overlaying the adenoma posteriorly. Finally, we routinely perform trigonization, involving anastomosis of the bladder mucosa to the posterior aspect of the urethra covering the prostastic capsule, to achieve perfect hemostasis and reduce the risk of bladder neck contracture. Although this surgical step is not described in the report by Leslie et al, it is demonstrated in one of the three cases included in the video accompanying their paper, suggesting that the University of Southern California urologic team also performs this step, at least in some patients.
Our study is important for several reasons. First, it involved accurate collection and reporting of data on postoperative complications in accordance with the Martin criteria [20] . The accuracy of data reporting might partly explain the relatively high number of low-grade complications reported, as previously suggested for other urologic procedures [21] . Conversely, it is also possible that complication rates are affected by surgeon experience with the procedure, as suggested by the findings of our multivariate analysis. Although both surgeons were experienced in robotic techniques before performing RASP, the present series includes the learning curves of both surgeons for the procedure. It can be hypothesized that some postoperative bleeding that required a return to theatre for cystocoagulation might have been avoided with greater RASP experience. However, our complication rate compares favorably with the overall rate of 20% reported by Leslie et al [7] , which included 8% grade 2, 8% grade 3a, and 4% grade 3b complications. Second, although the number of events was small and the statistical analyses were affected by low statistical power, we were able to identify some predictors of complications, including age and surgeon experience with the procedure. Although these findings might seem obvious, predictors of RASP complications have never been reported before and the findings might be useful for patient counseling and selection. Third, the median catheterization time (3 d) in our cohort compares favorably with those reported for OP series and many other smaller RASP series, for which catheterization time ranged from 4.8 to 13 d[10] and [11]. This reflects our policy to keep patients in hospital until early catheter removal and free voiding rather than being discharged with a catheter. Despite the early catheter removal, we have not experienced any problems with leakage from the cystotomy closure, which we attribute to careful technique and the use of a double-layer 3-0 V-Loc closure of the bladder wall. Our current policy is to remove the catheter once clear urine is observed, even on postoperative day 1 or 2; thus, a quarter of our patients had a catheterization time of ≤2 d.
There are several limitations to our study. First is the limitation inherent to retrospective analyses. Moreover, the sample size is relatively small and procedures were performed by two different surgeons with different levels of robotic expertise. The patient cohort includes all cases performed in our center since the inception of the procedure. Consequently, the learning curve of the two surgeons might have affected both complications and functional outcomes. However, the number of complications (especially those of high grade) was relatively limited, although this might have limited our ability to identify predictors. Moreover, our follow-up is quite short, which precludes us from assessing even intermediate-term outcomes. This is mainly because our hospital is a referral center for robotic procedures and patients are always seen within 6 wk after surgery, but often continue functional follow-up with their referring urologist. Besides, we did not study the prevalence of erectile dysfunction. Finally, the study design does not allow us to draw any conclusions on RASP effectiveness and costs in comparison with HOLEP or laparoscopic simple prostatectomy.
Our data support RASP as a safe and effective surgical technique for treating patients with LUTS with large prostate adenoma. Our data show an excellent improvement in patient symptoms and flow scores at short-term follow-up, with an acceptable risk profile. We recommend RASP as a very useful skill in the armamentarium of surgeons already acquainted with robotic radical prostatectomy as an alternative to open simple prostatectomy. The effectiveness of the procedure in comparison with other endoscopic treatments for large adenomas (especially HOLEP) has to be established in comparative studies.
Author contributions: Giacomo Novara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Novara, Schatteman, Mottrie.
Acquisition of data: Pokorny, Geurts, Dovey, De Groote.
Analysis and interpretation of data: Novara, De Naeyer, Mottrie.
Drafting of the manuscript: Pokorny, Novara.
Critical revision of the manuscript for important intellectual content: Pokorny, Novara, Geurts, Dovey, De Groote, Ploumidis, Schatteman, de Naeyer, Mottrie.
Statistical analysis: Novara.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Mottrie.
Other: None.
Financial disclosures: Giacomo Novara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.