Context
Treatment for lower urinary tract symptoms resulting from benign prostatic hyperplasia (BPH) is varied, and significant side effects, particularly concerning sexual function, affect uptake. The prostatic urethral lift (PUL) procedure is a recent addition to the armamentarium for BPH treatment, with independent reports suggesting improvement of symptoms, sexual function, and urinary flow.
Objective
We undertook a systematic review and meta-analysis of reported symptomatic, functional, and sexual outcomes following the PUL procedure.
Evidence acquisition
We performed a critical review of Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science databases in May 2014 according to the Preferred Reporting Items for Systematic Review and Meta-Analysis statement. Quality assessment was performed using a modification of the Methodological Index for Non-Randomized Studies tool. All retrospective, prospective, and controlled trials were included for analysis. Symptom scores, sexual health scores, and functional outcomes were pooled and meta-analysed using quality and random-effects models.
Evidence synthesis
Ten articles comprising six independent patient cohorts were included for analysis. Pooled estimates from between 452 and 680 patients suggested overall improvement following PUL, including symptoms (large gain; standardised mean gain range of 1.3–1.6, International Prostate Symptom Score difference of −7.2 to −8.7 points), maximum flow rate (3.8–4.0 ml/s), and quality of life (2.2–2.4 points). Sexual function was preserved with a small improvement estimated at 12 mo (standardised mean gain range of 0.3–0.4). Pooled estimates were mostly heterogeneous across study groups.
Conclusions
PUL is a well-tolerated, minimally invasive therapy for BPH that provides favourable symptom, sexual health, and functional outcomes during follow-up to 12 mo. Longer follow-up and larger randomised studies are required to further confirm these preliminary results.
Patient summary
We reviewed the early results of an innovative procedure directed towards the management of prostate enlargement. The results revealed a well-tolerated procedure that produces improvement in urinary symptoms and function while preserving sexual function.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.
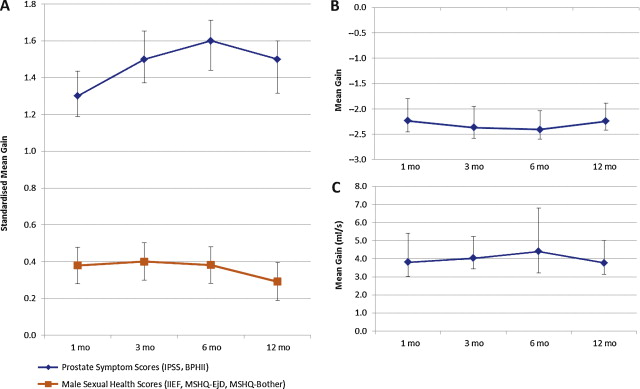
The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.
Lower urinary tract symptoms (LUTS) resulting from benign prostatic hyperplasia (BPH) are common, with moderate to severe LUTS estimated to affect up to 30% of men aged >50 yr[1] and [2]. Severe LUTS is associated with depression and reduced quality of life in otherwise healthy men [3] , and an increasing population requiring treatment is expected, specifically 10.3 million men in the United States in 2020[1] and [4]. Reduced healthcare-related quality of life causes significant economic burden [5] . Current methods of conservative treatment (α1-blockers, 5α-reductase inhibitors) total 11.6 million prescriptions per year across Europe [6] for modest improvements in the International Prostate Symptom Score (IPSS).
Up to 30% of patients require surgical intervention following failure of medical therapy, mostly due to dissatisfaction and side effect profile[1] and [7]. Transurethral resection of the prostate (TURP) produces a significant and reliable improvement in LUTS as a result of reducing bladder outlet obstruction [8] at the expense of morbidity, such as ejaculatory dysfunction (53–75%), erectile dysfunction (3.4–32%), urinary incontinence (2.2%), and urethral stricture (2–9%) [9] . Despite alternative therapeutic advances such as photoselective vaporisation of the prostate (PVP), the side effect profile remains prominent, with 8.8% suffering perioperative complications and 13.3% having long-term morbidity with this procedure[10] and [11]. Less invasive, orminimally invasive, surgical interventions such as transurethral microwave therapy (TUMT) and transurethral needle ablation (TUNA) spare a degree of the side effect profile at the expense of IPSS improvement [10] .
Prostatic urethral lift (PUL) is a newly available minimally invasive procedure for LUTS secondary to BPH. Despite being categorised as minimally invasive, PUL is performed in the lithotomy position with the support of a local or general anaesthetic. Initially reported in 2005, the procedure results in anterolateral traction of the lateral lobes of the prostate towards the capsule, expanding the urethral lumen and relieving obstruction [12] . The procedure has been described in detail previously [13] . The lateral lobes are secured by small permanent suture-based implants administered by a preloaded custom implant-delivery device (UroLift System; NeoTract Inc., Pleasanton, CA, USA). Given this targeted mechanism on the lateral lobes, it has been postulated that PUL may have limited efficacy for patients with obstructing median lobes, which has been an important exclusion criterion for many previously published reports. This interventional technique is mechanical and avoids resection or ablation of prostatic tissue. Early results using PUL suggest a beneficial therapeutic effect while avoiding many of the morbidities and complications associated with more conventional surgery.
The aim of this study was to collate available data on PUL using a systematic search strategy and to quantify global treatment effects using meta-analysis. This article was produced without consultation or input from NeoTract Inc.
A systematic review was performed in accordance with Cochrane Collaboration and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines[14] and [15].
Scientific literature databases (Medline, Embase, ScienceDirect, Cochrane Library, and Web of Science) were systematically searched in May 2014 using several keywords includingprostate, benign prostate hyperplasia, lower urinary tract symptoms, andurethral lift(Supplementary Table 1). Article selection was performed by two independent evaluators (M.P., M.J.R.), and any discrepancies were resolved.
Retrospective and prospective studies evaluating functional and sexual outcomes following the PUL procedure for LUTS resulting from BPH were included in the analysis. No language or sample-size restrictions were used. Conference proceedings were unable to be robustly assessed for study quality and thus were excluded. If duplicate study populations or analyses of repeated data were identified, the publication reporting a larger sample size was preferentially assessed.
Initially, studies were quality assessed based on the Cochrane Handbook for Systematic Reviews of Interventions 5.02[15] and [16]. A quality-appraisal tool was adapted for the current research question from the recommendations by Ramsey et al [17] . Each paper was scored independently by two evaluators (M.J.R, M.P.), and these scores were used to appropriately weight each study when performingquality effectsmeta-analysis [18] (Supplementary Table 2).
Data extracted from the eligible studies included demographic information (eg, patient age, prostate volume), operative details (eg, number of implants, operative time, perioperative complications), and postoperative outcomes. Primary outcome measures that were assessed included prostate symptoms (IPSS, American Urological Association Symptom Index, BPH Impact Index [BPHII]), sexual health (International Index of Erectile Dysfunction [IIEF], Sexual Health Inventory for Men, Male Sexual Health Questionnaire [MSHQ] for ejaculatory function [MSHQ-EjD] and bother [MSHQ-Bother]), and functional parameters including maximum urine flow rate (Qmax) and postvoid residual volume (PVR).
Extracted data were collated in Excel (Microsoft Corporation, Redmond, WA, USA). Quality and random-effects meta-analysis was performed using MetaXL 2.0[18], [19], and [20]( http://www.epigear.com ). If results from one scoring system (eg, quality of life score, Qmax, PVR) were pooled, the effect size extracted was the gain in mean score or mean gain (MG) plus or minus standard error (SE) from before (time point 1 [T1]) to after (time point 2 [T2]) the intervention. The following calculations were used:
FORMULA:

in which
If multiple scales were used to measure the same outcome, a standardised MG (SMG) was calculated by considering the MG in the context of the pooled standard deviation (sp) [21] . The following calculations were used:
FORMULA:

in which
The interpretation of SMG is similar to Cohen'sd [22] , for which small, medium, large, and very large effect-size thresholds are defined as 0.2, 0.5, 0.8, and 1.3, respectively. To aid in clinical understanding, interpretation of the SMG as a difference in IPSS (the IPSS is a 35-point scale with 7 points being a large difference) was undertaken by multiplying the SMG and its 95% confidence interval (CI) by 5.5, which represents a typical standard deviation for the IPSS scores. This interpretation should be considered indicative only.
Random-effects model results were reported only in Supplementary Table 3 for comparison purposes because the results of this model are known to underestimate the statistical error and defaults to the arithmetic mean when heterogeneity is large. It also ignores the risk of bias from individual studies; as such, results that differ from the quality effects model results may not be meaningful. Heterogeneity in effect sizes was defined as τ2greater than zero or Q-statistic >50 [23] .
Using the systematic search strategy outlined in Supplementary Table 1, 581 articles were identified, of which 58 were duplicate records that were excluded ( Fig. 1 ). Of the remaining 523 records, 490 were not relevant to the research question and 23 were conference abstracts that could not be quality assessed and thus were excluded. From the remaining 10 articles, 6 independent patient series were identified for analysis. Of these six, one represented a randomised controlled trial[24] and [25], one was an observational crossover cohort from the placebo group in the randomised controlled trial [26] , two were prospective trials [27] , and two were retrospective cohorts[4], [13], [28], [29], and [30]( Table 1 ). Patient demographics and baseline symptom, functional, and sexual measures are outlined in Table 2 . Five studies were included in the meta-analysis because one study did not report standard deviations and was excluded [28] .

Table 1 Characteristics of included studies
| Series | Study type | Country | n | Inclusion criteria | Exclusion criteria | Follow-up (mo) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | Prospective cohort | Spain | 20 | Aged >50 yr, IPSS >20, Qmax <15 ml/s, no prior BPH treatment | PSA >10, obstructive median lobe, infection, previous prostate surgery | 12 | IPSS, BPHII, Qmax |
| Cantwell et al, 2014 [26] | Crossover trial | USA | 53 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother |
| Delongchamps, cited in Hoffman et al, 2012 [49] | Prospective cohort | France | 4 | NR | NR | 1 | IPSS, SHIM, MSHQ-EjD |
| LIFT study, Roehrborn et al, 2013 [24] | Blinded RCT | USA | 140 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, retention, PVR >250 ml, infection, PSA >10 (unless negative biopsy), cystolithiasis, bacterial prostatitis | 12 | AUASI, QOL, BPHII, Qmax, PVR |
| LIFT study, McVary et al, 2014 [25] | Blinded RCT | USA | 137 | As above | As above | 12 | IPSS, SHIM, MSHQ-EjD, Qmax |
| Shore et al, 2014 [27] | Prospective cohort | USA | 51 | Aged >50 yr, no prior BPH treatment, washed out or naive to medical therapy, IPSS >12, Qmax <12 ml/s, PV 30–80 ml | Obstructive median lobe, PVR >250 ml, infection, cystolithiasis, bacterial prostatitis | 1 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, MSHQ-Bother, Qmax |
| McNicholas et al, 2013 [13] | Prospective cohort | UK | 102 | PV <60 ml, IPSS >12, Qmax <15 ml/s, PVR <250 ml | NR | 12 | IPSS, QOL, BPHII, Qmax, PVR |
| Chin et al, 2012 [30] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml | PSA >10, retention, infection, previous prostate surgery, large median lobes, compromised renal function | 24 | IPSS, QOL, BPHII, SHIM, MSHQ-EjD, Qmax, PVR |
| Woo et al, 2012 [29] | Prospective cohort | Australia | 64 | IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, retention, infection | 12 | IPSS, SHIM, MSHQ-EjD, MSHQ-Bother |
| Woo et al, 2011 [4] | Prospective cohort | Australia | 15 | PV 20–100 ml, IPSS >12, Qmax 5–12 ml/s, PVR <250 ml, washed out to medical therapy | Obstructive median lobe, infection, retention, PSA >10, significant medical comorbidities, previous surgery | 12 | IPSS, QOL, Qmax, PVR |
AUASI = American Urological Association Symptom Index; BPH = benign prostatic hyperplasia; BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; QOL = quality of life; PSA = prostate-specific antigen; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SHIM = Sexual Health Inventory for Men.
Table 2 Baseline functional scores prior to prostatic urethral lift intervention
| Series | n | Age | PV, ml, mean (SD) | IPSS, mean (SD) | BPHII, mean (SD) | HRQOL, mean (SD) | IIEF-5 or SHIM, mean (SD) | MSHQ-EjD, mean (SD) | MSHQ-Bother, mean (SD) | Qmax, ml/s, mean (SD) | PVR, ml, mean (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abad et al, 2013 [28] | 20 | 74.3 ± NR | 42.6 (NR) | 26.7 (6.0) | 8.4 (2.3) | NR | NR | NR | NR | 8.6 (2.9) | NR |
| Cantwell et al, 2014 [26] | 53 | 64 ± 8.0 | 40.3 (9.9) | 23.3 (5.5) | 6.3 (3.0) | 4.5 (1.2) | 12.8 (8.3) | 9.5 (10.0) | NR | 8.8 (4.2) | 67.8 (66.4) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | 4 | 63.5 ± 11.1 | 55 (19) | 20.8 (3.1) | NR | NR | NR | 5.5 (6.1) | NR | 6 (2.3) | 115 (72) |
| LIFT study, Roehrborn et al, 2013 [24] | 140 | 67 ± 8.6 | 44.5 (12.4) | 22.2 (5.4) | NR | 4.6 (1.1) | 13.0 (8.4) | 8.7 (3.2) | NR | 8.9 (2.2) | 85.5 (69.2) |
| LIFT study, McVary et al, 2014 [25] | 137 | 67 ± NR | NR | 22.2 (5.4) | NR | 4.6 (1.1) | 18.0 (5.6) | 9.1 (3.1) | 2.0 (1.6) | 8.0 (2.4) | NR |
| Shore et al, 2014 [27] | 51 | 66 ± 7.6 | 41.3 (11.6) | 21.5 (5.4) | 6.7 (3.1) | 4.6 (1.0) | 16.5 (7.3) | 9.95 (2.6) | NR | 8.2 (2.2) | 77.1 (74.9) |
| McNicholas et al, 2013 [13] | 102 | 68 ± 10.0 | 48 (10.5) | 23.2 (6.1) | NR | 4.7 (1.0) | NR | NR | NR | 8.7 (4.0) | NR |
| Chin et al, 2012 [30] | 64 | 67 ± 7.3 | 51 (11.5) | 22.6 (5.4) | NR | NR | NR | NR | NR | 8.3 (2.2) | NR |
| Woo et al, 2012 [29] | 64 | 67 ± 7.3 | 51 (11.5) | 22.9 (5.4) | NR | NR | 11.7 (8.6) | 9.0 (3.7) | 1.7 (1.5) | NR | NR |
| Woo et al, 2011 [4] | 15 | 66 ± 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
BPHII = Benign Prostatic Hyperplasia Impact Index; EjD = ejaculatory dysfunction; HRQOL = health-related quality of life; IIEF-5 = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire; NR = not reported; PV = prostate volume; PVR = postvoid residual; Qmax = maximum flow rate; RCT = randomised controlled trial; SD = standard deviation; SHIM = Sexual Health Inventory for Men.
Patient inclusion and exclusion criteria remained relatively constant across study groups, with patients aged >50 yr with IPSS >12 and Qmax<12–15 ml/s. Accepted prostate volumes varied between studies but generally included patients with volumes 20–100 ml, with a cut-off of ≤80 ml applied in the randomised controlled trial. The most consistently reported exclusion criteria were obstructive median lobes, active urinary infection, acute urinary retention (or PVR >250 ml), and prostate-specific antigen (PSA) >10 ng/ml (unless normal biopsy). Patient demographics across the six series were comparable, with a median age range of 64–74.3 yr and mean operative time range of 19.1–66 min. Anaesthesia methods varied, with most US-based series completed under local anaesthetic, with or without penile block (95–99%). The procedure was well tolerated across all series, with a serious complication rate (defined as a Clavien-Dindo [31] score of ≥2) of 2.5–18%. As shown in Table 3 , the most frequent complications reported within 3 mo postoperatively included dysuria (25–53%), haematuria (16–75%), pelvic pain (3.7–19.3%), urgency (7.8–10%), transient incontinence (1.9–16%), and urinary tract infection (3.2–10%). Varied definitions and terminology limited statistical comparison.
Table 3 Operative details and complications
| Series | Local anaesthetic | Operative time, min, mean (SD) | Implants, mean (range) | Postoperative catheter | Early postoperative complications | Progression to TURP at 12 mo | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dysuria | Haematuria | Pelvic pain | UTI | Incontinence | ||||||
| Abad et al, 2013 [28] | 0/20 (0) | 19.1 (NR) | 3.8 (2–6) | NR | 14/20 (70) | 6/20 (30) | NR | NR | 0/20 | 1/20 (5) |
| Cantwell et al, 2014 [26] | 46/53 (88) | 53 (15) | 4.4 (2–8) | 26/53 (49) | 19/53 (36) | 14/53 (26) | 11/53 (21) | 1/53 (2) | 2/53 (3.8) | 1/53 (2) |
| Delongchamps, cited in Hoffman et al, 2012 [49] | NR | 11 (4.7) | 5.5 (3–9) | 4/4 (100) | 0/4 (0) | 1/4 (25) | 0/4 (0) | 0/4 (0) | 0/4 (0) | NA |
| LIFT study, Roehrborn et al, 2013 [24] | NR | 66 (24) | 4.9 (2–11) | 72/140 (51) | 48/140 (34) | 36/140 (26) | 25/140 (18) | 4/140 (2.9) | 5/140 (3.6) | 2/140 (1.4) |
| LIFT study, McVary et al, 2014 [25] | NR | NR | 4.9 (NR) | 72/137 (53) | NR | NR | NR | NR | NR | 2/137 (1.5) |
| Shore et al, 2014 [27] | 51/51 (100) | 52 (22) | 3.7 (2–6) | 10/51 (20) | 27/51 (53) | 38/51 (75) | 8/51 (16) | NR | 2/51 (4) | NA |
| McNicholas et al, 2013 [13] | 17/102 (17) | 58 (16) | 4.5 (2–9) | 43/102 (42) | 25/102 (25) | 16/102 (16) | NR | 3/102 (3) | NR | 4/102 (3.9) |
| Chin et al, 2012 [30] | 26/64 (41) | NR | NR | 34/64 (53) | NR | NR | NR | 7/64 (11) * | 5/64 (8) | 12/64 (19) * |
| Woo et al, 2012 [29] | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Woo et al, 2011 [4] | 0/15 (0) | NR | 3.5 (2–5) | 11/19 (58) | 11/19 (58) | 12/19 (63) | 1/19 (5) | 3/19 (10) † | 3/19 (16) | 3/19 (16) |
* Over 24-mo follow-up period.
† Over 12-mo follow-up period.
NA = not available; NR = not reported; SD = standard deviation; TURP = transurethral resection of the prostate; UTI = urinary tract infection.
Data are shown as frequency (%) unless indicated otherwise.
The pooled SMG estimates for prostate symptom scores (IPSS and BPHII) and sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) incorporated between 888 and 1298 responses from 452 to 680 patients. The SMG for prostatic symptom scores ranged between −1.3 (95% CI, −1.4 to −1.2) and −1.6 (95% CI, −1.7 to −1.3), suggesting a large decrease in symptoms across the 12-mo follow-up period ( Table 3 ; Fig. 2 A). Interpretation of the SMG in terms of difference in IPSS score suggests that the change in score was −7.2 points (95% CI, −7.9 to −6.5) at 1 mo, −8.3 points (95% CI, −9.1 to −7.5) at 3 mo, −8.7 points (95% CI, −9.4 to −7.9) points at 6 mo, and −8.0 points (95% CI, −8.8 to −7.2) at 12-mo follow-up. Prostate weight, number of implants, urethral length, degree of median lobe obstruction, and other prognostic factors identifying patients likely to have a greater therapeutic effect have not been reported and thus were not available for analysis.

The SMG in sexual health scores ranged between 0.3 (95% CI, 0.2–0.4) and 0.4 (95% CI, 0.3–0.5), suggesting a small improvement [22] . Pooled estimates were mostly heterogeneous, with some homogeneity observed (prostate symptom score at 6 mo, sexual health score at 12 mo).
Mean improvement in quality-of-life scores were estimated to be between 2.2 points (95% CI, −2.5 to −2.0) and 2.4 points (95% CI, −2.6 to −2.2) (MG) using responses from between 452 and 628 patients ( Table 3 ; Fig. 2 B). A homogeneous pooled estimate from three studies was observed for data at 12 mo, with the remainder being heterogeneous.
Assessment of functional outcomes (Qmax, PVR) was limited due to inconsistent reporting, specifically at selected intervals in selected studies. Nevertheless, favourable pooled estimates were observed for Qmax, with an improvement of between 3.8 ml/s (95% CI, 3.0–4.6) and 4.0 ml/s (95% CI, 3.4–4.6) observed in meta-analysed studies (1, 3, and 12 mo), which were homogeneous ( Table 4 ; Fig. 2 C). Pooled PVR estimates were significantly variable, owing to inconsistent reporting, and demonstrated very high heterogeneity estimates ( Table 4 ). Figure 2 also indicates that effects due to the intervention were seen early and were sustained over 12 mo.
Table 4 Pooled estimates of outcome measures following the prostatic urethral lift procedure
| 1 mo | 3 mo | 6 mo | 12 mo | |
|---|---|---|---|---|
| Prostate symptom scores (IPSS, BPHII) | ||||
| No. of data sources, response sample size (n) | 9 (1298) | 6 (1050) | 6 (1022) | 6 (888) |
| Effect size (95% CI) | −1.30 (−1.4 to −1.2) | −1.50 (−1.7 to −1.4) | −1.6 (−1.7 to −1.3) | −1.5 (−1.6 to −1.3) |
| Heterogeneity (τ2) | 0.1 | 0.1 | 0.00 | 0.00 |
| Male sexual health scores (IIEF, MSHQ-EjD, MSHQ-Bother) | ||||
| No. of data sources, response sample size (n) | 13 (1042) | 9 (889) | 9 (908) | 9 (786) |
| Effect size (95% CI) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.2–0.4) |
| Heterogeneity (τ2) | 0.00 | 0.00 | 0.00 | 0.00 |
| Health-related quality of life | ||||
| No. of data sources, response sample size (n) | 4 (628) | 3 (508) | 3 (496) | 3 (452) |
| Effect size (95% CI) | −2.2 (−2.5 to −2.0) | −2.4 (−2.6 to −2.2) | −2.4 (−2.6 to −2.2) | −2.2 (−2.4 to −2.1) |
| Heterogeneity (τ2) | 0.2 | 0.1 | 0.1 | 0.00 |
| Maximum flow rate | ||||
| No. of data sources, response sample size (n) | 3 (242) | 3 (488) | 1 * (106) | 3 (362) |
| Effect size (95% CI) | 3.8 (3.0–4.6) | 4.0 (3.4–4.6) | 4.4 (3.2–5.6) | 3.8 (3.1–4.4) |
| Heterogeneity (τ2) | 0.4 | 0.03 | NA | 0.2 |
| Postvoid residual | ||||
| No. of data sources, response sample size (n) | 2 (128) | 2 (396) | 1 * (122) | 2 (350) |
| Effect size (95% CI) | 15.5 (12.6–18.6) | −6.2 (−10.1 to −2.8) | −11 (−13 to −9) | −4.0 (−10.5 to 2.6) |
| Heterogeneity (τ2) | 1732 | 24 | NA | 219 |
* Individual study data reported, no meta-analysis performed.
BPHII = Benign Prostatic Hyperplasia Impact Index; CI = confidence interval; EjD = ejaculatory dysfunction; IIEF = International Index of Erectile Function; IPSS = International Prostate Symptom Score; MSHQ = Male Sexual Health Questionnaire.
At 12-mo follow-up, 1.5–16% of patients experienced insufficient IPSS or Qmaximprovements and progressed to TURP. Several studies reported results of routine follow-up cystoscopic evaluation of the PUL implants. At 6 mo postoperatively, no study reported implant encrustation or removal of troublesome implant. At 12-mo follow-up, no appropriately placed implants developed encrustation; however, 14 of 27 implants exposed to the bladder experienced encrustation, and 2 of 14 were symptomatic and were removed with endoscopic forceps[24] and [25]. Implant failure or infection requiring removal was not reported.
PUL is a well-described, minimally invasive addition to the armamentarium for BPH treatment. The results of the current systematic review and meta-analysis suggest that PUL produces improved prostatic symptoms and urinary function with relative preservation of sexual function.
A large [22] improvement of LUTS symptoms was observed in the pooled analysis of symptom scores (IPSS and BPHII). When estimated on the IPSS scale, an improvement of −8.0 points (95% CI, −8.8 to −7.2) was estimated at 12-mo follow-up. The mechanical improvement resulting from superolateral traction of the lateral prostatic lobes provided by the PUL procedure is estimated to be more effective than medical therapies and placebo, which both improve IPSS at 12 mo by 3.5–7.5 points [1] . Alternative minimally invasive therapies, including TUNA and TUMT, produce similar improvements in IPSS of 9.3–12.4 points at 12-mo follow-up when compared with the PUL procedure[10] and [32]. Both TURP and PVP have been shown to produce excellent improvements in IPSS at 12 mo of up to 14.9 points[10], [33], and [34]but have operation-associated morbidity and inpatient hospital costs. Compared with TURP and PVP, PUL is minimally invasive without a requirement of general anaesthesia and with potentially shorter operative time, which may provide cost benefits, although these have not been assessed to date. Furthermore, the long-term durability of PUL in improving IPSS is uncertain in the published literature, with maintained outcomes reported at 24-mo follow-up in a single study [30] .
Functional benefits of PUL were also observed in the pooled estimates, specifically, improvements of up to 3.80 ml/s in Qmaxduring 1 mo and 12 mo in three studies; however, this analysis of the early outcomes of the PUL procedure demonstrated heterogeneity for Qmaxoutcomes. Consequently, the values obtained are not suitable for direct comparison with alternative therapies, and the resulting improvements in Qmaxshould be considered with caution. The functional improvements observed in the current meta-analysis appear noninferior when compared with current medical[1] and [35]and minimally invasive[36], [37], and [38]therapies. Functional improvements following PUL are fewer than those following surgical interventions including TURP and PVP, which are associated with an improved Qmaxof between 10 and 13 ml/s[33], [34], [39], and [40]at 12-mo follow-up. Significant heterogeneity retards the comparison of PVR estimates, for which benefits were also observed following sham procedures (with associated cystoscopy), presumably as result of urethral dilation resulting from both approaches. Current evidence suggests that the currently described PUL procedure requires alteration to improve functional performance for equivalence with surgical interventions.
Sexual health outcomes in the current meta-analysis included five cohorts reporting IIEF, MSHQ-EjD, and MSHQ-Bother in patients undergoing PUL. Pooled estimates of overall sexual function scores suggested a consistently small improvement [41] throughout follow-up ( Table 3 ) that is favourable compared with medical and surgical alternatives. Because LUTS is well established as an independent risk factor for sexual dysfunction[42], [43], and [44], precipitation or exacerbation of sexual dysfunction commonly complicates medical and surgical treatment. Impact on sexual function following medical therapies (α1-blockers, 5α-reductase inhibitors) has been reported inconsistently but generally is considered to be due to loss of libido with erectile and ejaculatory dysfunction[25], [35], [45], [46], [47], and [48]. Minimally invasive therapies, including TUMT and TUNA, are characterised by increased risk of erectile dysfunction (0–18.2%) and retrograde ejaculation (9.2–22.2%)[49] and [50]. More invasive options, including TURP or PVP, are associated with high rates of erectile dysfunction (14–26%) and ejaculatory dysfunction (15–63%)[10], [50], and [51]. The causative mechanism for these side effects is unclear, with hypotheses including diathermy-induced autonomic nerve injury and injury to the bladder neck or musculus ejaculatorius[10], [11], [52], and [53]. Regardless of the hypothesis, these vital structures are prone to functional compromise following treatment with ablative modalities. Improved sexual function following the preservation of native prostatic tissue achieved with the PUL technique is a key benefit of this minimally invasive treatment alternative for LUTS secondary to BPH.
PUL is a novel, minimally invasive treatment modality for LUTS secondary to BPH. The targeted mechanism on the lateral lobes leaves the median lobe relatively unchanged following the implantation of this device. Intuitively, patients with obstructing median lobes may receive limited therapeutic benefit. To date, all prospective trials are characterised by the exclusion of patients with obstructing median lobes. As such, the effect in such patients has not been studied and is largely unknown. Similarly, other variables including high prostate volumes and long prostatic urethral length may limit the benefit obtained. Such variables have not been assessed in the current literature and represent the scope for further research. The use of PUL in patients with elevated PSA has been addressed, with many studies outlining a preoperative PSA >10 ng/ml as a relative exclusion criteria for the PUL procedure[13], [24], [25], and [30]; however, several studies subsequently include these patients following normal prostatic biopsy and report relative safety.
Further prostatic intervention following implementation of the PUL implants has been considered to the extent possible in the available literature. Published series’ report extrusion into the bladder lumen and subsequent encrustation of bladder neck implants, a vast majority of which were asymptomatic and managed conservatively. At 24-mo follow-up, only one series documented the removal of implants in the context of significant encrustation. Progression to TURP or PVP following the PUL procedure was reported, outlining the ability of the resectoscope to instantaneously melt the monofilament implants without problems[4] and [30]. The presence of implants was reported to have no influence on surgical routine during TURP or PVP. No publication to date has reported outcomes following investigation or management of a subsequent diagnosis of prostate cancer, including prostatic biopsy, brachytherapy, or prostatectomy after the PUL procedure.
As with any therapeutic intervention, benefits secondary to placebo may confound results. To date, a sole multicentre randomised controlled trial for the PUL procedure has been published[24] and [25]. The aforementioned study performed a sham procedure during cystoscopy, which allowed the postoperative physiologic changes associated with cystoscopic dilation of the urinary tract to be differentiated from the PUL. The resulting symptom scores and functional measures following PUL were superior within 3 mo postoperatively [24] . Sexual function and satisfaction scores improved following PUL but did not differ significantly within 3 mo from the placebo-control cohort [25] . An inability to maintain long-term benefits may be due to cystoscopic dilation as well as to the placebo effect. Study participants were blinded until 3 mo postoperatively and then subsequently offered PUL, with these results reported as a crossover-type analysis [26] . It is clear that further research is required with prolonged follow-up of placebo-control and study participants over the prior 6 mo and with more participants.
The ultimate place of PUL in the management of BPH has yet to be determined. The ability of this technique to provide symptom benefits following a single short procedure, without the risk of retrograde ejaculation, positions it as a potential alternative to both medical and more invasive surgical procedures for which this side effect is more prominent. Currently it would seem to have a justifiable role in the management of men with moderate BPH (prostate volume <80 g) for whom the potential for this side effect as a consequence of treatment would either reduce their quality of life or lead them to defer intervention even at the risk of more significant complications. Longer term evaluation (>3 yr of data) of durability and symptomatic improvement produced by PUL will play a key role in determining its ultimate place in the management of BPH.
The present meta-analysis has several limitations. Limited published reports examining this procedure required that all studies, of varying quality, be included in the meta-analysis. As such, the inherent high risk of bias was adjusted for use of quality scoring of individual studies, which was statistically incorporated into the quality effects pooled estimates. Further limitations owe to the naïve standing of the PUL procedure for treatment of LUTS secondary to BPH because this allowed meta-analysis of effect sizes only at 12-mo follow-up. Thus, the long-term durability of this device cannot be commented on appropriately. In light of these limitations of the studies and the respective results assessed with appropriate adjustment, a high degree of heterogeneity was obtained. This was particularly evident in pooled analysis of overall symptoms and sexual function. Furthermore, publication bias and favourable reporting owing to commercial interests with the current method of PUL cannot be discounted.
We identified five independent series evaluating the symptomatic, sexual, and functional outcomes following PUL. Our results suggest that this procedure is associated with minimal perioperative morbidity, whereas meta-analysis estimates suggest improvements in symptomatic and functional outcomes that are durable through 12-mo follow-up. Preservation of the bladder neck and subsequent control of sexual function following PUL provide stark contrast to the medical and surgical alternatives for treatment of BPH. Further comparative trials with longer follow-up periods and cost–benefit analyses are required to guide clinicians as to the suitability of PUL in routine clinical practice.
Author contributions: Damien Bolton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Perera, Roberts, Doi.
Acquisition of data: Perera, Roberts.
Analysis and interpretation of data: Roberts, Doi.
Drafting of the manuscript: Perera, Roberts, Bolton.
Critical revision of the manuscript for important intellectual content: Doi, Bolton.
Statistical analysis: Roberts, Doi.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Bolton.
Other(specify): None.
Financial disclosures: Damien Bolton certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: M.J.R. is supported by a Doctor in Training Research Scholarship from Avant Mutual Group Ltd., Cancer Council Queensland PhD Scholarship and Professor William Burnett Research Fellowship from the Discipline of Surgery, School of Medicine, The University of Queensland.
Funding/Support and role of the sponsor: None.
Acknowledgment statement: The authors are grateful to Professor RA “Frank” Gardiner (University of Queensland; Royal Brisbane and Women's Hospital, Australia) for critical revisions of the manuscript.