Background
Transurethral resection of the prostate (TURP) is considered the gold standard for male lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH). However, TURP may lead to sexual dysfunction and incontinence, and has a long recovery period. Prostatic urethral lift (PUL) is a treatment option that may overcome these limitations.
Objective
To compare PUL to TURP with regard to LUTS improvement, recovery, worsening of erectile and ejaculatory function, continence and safety (BPH6).
Design, setting, and participants
Prospective, randomized, controlled trial at 10 European centers involving 80 men with BPH LUTS.
Intervention
PUL or TURP.
Outcome measurements and statistical analysis
The BPH6 responder endpoint assesses symptom relief, quality of recovery, erectile function preservation, ejaculatory function preservation, continence preservation, and safety. Noninferiority was evaluated using a one-sided lower 95% confidence limit for the difference between PUL and TURP performance.
Results and limitations
Preservation of ejaculation and quality of recovery were superior with PUL (p < 0.01). Significant symptom relief was achieved in both treatment arms. The study demonstrated not only noninferiority but also superiority of PUL over TURP on the BPH6 endpoint. Study limitations were the small sample size and the inability to blind participants to enrollment arm.
Conclusions
Assessment of individual BPH6 elements revealed that PUL was superior to TURP with respect to quality of recovery and preservation of ejaculatory function. PUL was superior to TURP according to the novel BPH6 responder endpoint, which needs to be validated in future studies.
Patient summary
In this study, participants who underwent prostatic urethral lift responded significantly better than those who underwent transurethral resection of the prostate as therapy for benign prostatic hyperplasia with regard to important aspects of quality of life.
Trial registration
ClinicalTrials.gov NCT01533038.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).
Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.
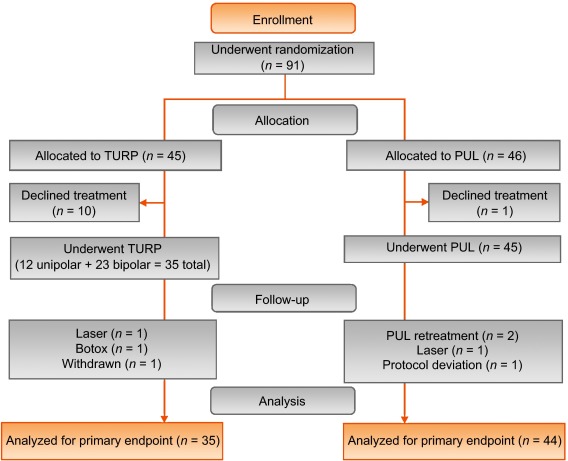
Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
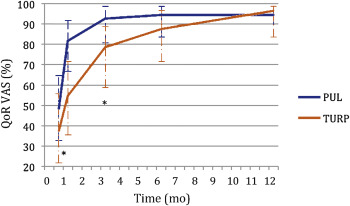
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).
Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).
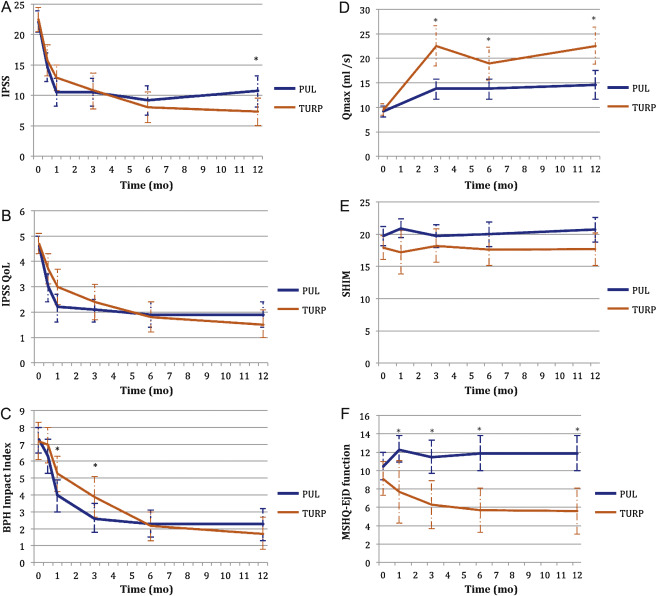
Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.
Male lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO) are a quality of life (QoL) issue estimated to affect 30% of men >50 yr of age, representing ∼26 million men in Europe [1] . Although symptom-relieving therapies exist, men may be hesitant in seeking invasive care because of the associated risks. In selecting treatment options for one QoL issue, LUTS, it may be important to consider other aspects of QoL. Studies that investigated the extent to which LUTS severity influences QoL indicate that the most important factors are often not changes in symptoms but the preservation of continence and sexual function [2], [3], and [4]. In addition to potency, ejaculatory function has been found to significantly influence sex life [5] . Patient satisfaction is also determined by return to normal activity and perioperative complications [6] . This study introduces a new, comprehensive endpoint referred to as BPH6 that reflects these patient-important goals: (1) adequate relief from LUTS; (2) high-quality recovery experience; (3) maintenance of erectile function; (4) maintenance of ejaculatory function; (5) maintenance of continence; and (6) avoidance of high-grade complications.
Transurethral resection of the prostate (TURP) is the gold standard with regard to symptom relief and improvements in urinary flow, but is associated with significant morbidity and long-term complications including stricture (7%), surgical revision (6%), significant urinary tract infection (4%), bleeding requiring blood transfusion (3%), incontinence (3%), transurethral resection syndrome (1%), erectile dysfunction (10%), and ejaculatory dysfunction (65%) [7] and [8]. Less invasive techniques strive to offer a meaningful therapeutic response with less morbidity than TURP. The prostatic urethral lift (PUL) is a minimally invasive procedure that yields effective treatment with little morbidity [9], [10], [11], [12], [13], [14], [15], and [16]. Here we present 1-yr results from a prospective, randomized trial comparing PUL to TURP in terms of the BPH6 endpoint. We hypothesized that when evaluated using the BPH6 composite endpoint, PUL is not inferior to TURP as a treatment option.
A prospective, randomized, nonblinded study was conducted across three European countries. Ethics committee approval was obtained at each site (Clinicaltrials.gov NCT01533038). Eligible men were aged at least 50 yr, were candidates for TURP, and were enrolled by investigators if they met the study criteria ( Table 1 ). Parallel randomization was conducted at a ratio of 1:1 at the time of the procedure, stratified by site, and performed using permuted blocks of various sizes chosen at random and concealed through a password-protected computer database.
Table 1 Patient selection criteria
| Inclusion criteria |
| • Willing to sign informed consent |
| • Male aged ≥50 yr |
| • International Prostate Symptom Score >12 |
| • Qmax ≤15 ml/s for 125-ml voided volume |
| • Post-void residual volume <350 ml |
| • Prostate volume ≤60 cm3 on ultrasound |
| • Sexually active within 6 mo before the index procedure |
| • Sexual Health Inventory for Men score >6 |
| • Positive response to MSHQ-EjD (excluding the response “Could not ejaculate”) |
| • Incontinence Severity Index score ≤4 |
| Exclusion criteria |
| • Active urinary tract infection at time of treatment |
| • Bacterial prostatitis within 1 yr of the index procedure |
| • Cystolithiasis within 3 mo of the index procedure |
| • Obstructive median lobe, as assessed via ultrasound and cystoscopy |
| • Current urinary retention |
| • Urethral conditions that may prevent insertion of a rigid 20F cystoscope |
| • Previous TURP or laser procedure, pelvic surgery or irradiation |
| • Prostate-specific antigen ≥10 ng/l, history of prostate or bladder cancer |
| • Severe cardiac comorbidities |
| • Anticoagulants within 3 d of the index procedure (excluding up to 100 mg acetylsalicylic acid) |
| • Other medical condition or co-morbidity contraindicative for TURP or PUL |
| • Unwilling to report sexual function |
Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; TURP = transurethral resection of the prostate; PUL = prostatic urethral lift.
PUL involves transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction [15] . Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeon experience with PUL varied from zero to 20 procedures before enrollment, whereas each surgeon had extensive prior experience with TURP. Licensed urologists trained and experienced in TURP conducted TURP procedures in accordance with their own normal standards and practices. A single surgeon at each site conducted between two and 19 procedures using general (86%), spinal (13%), or topical (1%, PUL only) anesthesia in the operating room. One site had two surgeons, each of whom performed four or more procedures. Patients were followed with visits at 2 wk and 1, 3, 6, and 12 mo.
The BPH6 primary study endpoint is a composite of six elements that assess overall outcome. The objective was to show that the success rate for PUL is not inferior to TURP in terms of the composite endpoint at 12 mo. Two modifications were made to the original element definitions to increase the quality and relevance of the analysis. In the original endpoint definition, the sexual function elements were assessed at a single time point, 12 mo. Because sexual activity can vary from month to month, both elements were modified to instead assess sustained effects during 12 mo. In addition, the majority of patients reported a return to preoperative activity by 1 mo on a separate questionnaire, yet scored >70 rather than >80 on the visual analog scale (VAS). The threshold for quality of recovery was thus lowered from 80 to 70 to address this correlation. The final BPH6 responder endpoint is achieved if a participant meets all six of the criteria as defined in Table 2 : LUTS relief, recovery experience, erectile function, ejaculatory function, continence, and safety.
Table 2 The six elements of the final BPH6 responder endpoint
| BPH6 element | Assessment requirement | Rationale |
|---|---|---|
| LUTS relief | Reduction of ≥30% in IPSS at 12 mo compared to baseline | Analysis of large-scale randomized trials indicates that 30% IPSS improvement is a suitable threshold for patient satisfaction and treatment acceptability [17] |
| Recovery experience | QoR VAS ≥70 by 1 mo | Postoperative return to normal activity is measured using a global QoR VAS ( Fig. 1 ) with significant convergent validity with the QoR Score [18] , a postoperative recovery outcome with content and construct validity [19] suitable for ambulatory surgery [20] . The threshold of 70% by 1 mo is chosen to reflect high-quality, rapid recovery |
| Erectile function | Reduction of <6 points for SHIM compared to baseline during 12-mo follow-up | SHIM is widely used to measure the severity of erectile dysfunction in clinical practice [21] . and >5 points has been used as the minimum clinically meaningful change [22] |
| Ejaculatory function | Response to MSHQ-EjD question 3 indicating emission of semen during 12-mo follow-up | Absence of ejaculate has been quantified using the four-item MSHQ-EjD [23] . Postoperative emission of semen is indicated by a “non-zero” response to the volume item of the questionnaire |
| Continence preservation | ISI score of ≤4 points at all follow-up intervals | The ISI consists of two questions on the frequency and amount of urinary leakage [24] and has been used in epidemiological surveys and clinical trials of LUTS treatment [2] and [25]. An incontinence threshold of ISI >4 [25] corresponds to the threshold for severe incontinence in the three-level index [24] |
| Safety | No treatment-related adverse event greater than grade I on the Clavien-Dindo classification system at any time during the procedure or follow up | The Clavien-Dindo classification of surgical complications has been validated in many fields including urology [26] and [27]. A threshold of grade II+ was selected to account for events that might significantly affect a patient's postoperative course, such as those requiring surgery, endoscopy, radiology, or supranormal pharmacology. If a patient pursues secondary treatment, the failure to respond is captured in the effectiveness element (#1) and not the safety element (#6); the patient is therefore censored from the safety element analysis at all subsequent time points |
LUTS = lower urinary tract symptoms; IPSS = International Prostate Symptom Score; QoR VAS = quality of recovery visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
The study was powered to establish noninferiority of PUL to TURP for noninferiority delta of 10% for the BPH6 primary endpoint. Performance estimates from the literature predicted that power of 80% would be achieved with enrollment of 62 participants, assuming the BPH6 success rate was 51% and 30% for PUL and TURP, respectively. Additional participants were enrolled to account for potential loss to follow up. Should noninferiority be achieved, superiority was to be tested with no alpha inflation, since this follows the testing methodology for hierarchical closed-form hypotheses. A value of p < 0.05 was defined as statistically significant. The success rates for the primary study endpoint were first tested for noninferiority using an exact test and then for superiority using a χ2 test. The exact method was used to establish 95% confidence intervals for the primary endpoint response.
Secondary analyses included comparison of treatment groups with respect to International Prostate Symptom Score (IPSS), IPSS QoL, BPH impact index (BPH II), peak flow rate (Qmax), Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ-EjD), and post-void residual volume (PVR). Changes from baseline for these measures were compared across treatment groups at each follow-up visit using analysis of covariance with the baseline score as a continuous covariate. The χ2 test and Fisher's exact test were used for categorical characteristics as appropriate. The null hypothesis for pad use concerned the probability of new pad use given BPH6 failure. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA) or StatXact (Cytel, Cambridge, MA, USA).

Between February 2012 and October 2013, 80 patients (45 PUL, 35 TURP) were enrolled in a prospective, randomized, controlled study at ten European centers ( Fig. 2 ). One patient was excluded from the analysis for violation of the active urinary retention exclusion criterion. Participants were well matched between the study arms, with no statistically significant differences in baseline parameters except for the MSHQ-EjD function score ( Table 3 ). After adjusting for any difference in baseline parameters between the enrollment arms, the conclusions for the primary endpoint and the sexual function analyses remained unchanged.

Table 3 Baseline characteristics and procedure details for study participants
| Characteristics | PUL (n = 44) a | TURP (n = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | n | Mean | SD | Range | n | |
| Age (yr) | 63 | 6.8 | 50–84 | 44 | 65 | 6.4 | 51–78 | 35 |
| Prostate volume (cm3) | 38 | 12 | 16–59 | 44 | 41 | 13 | 17–68 | 35 |
| Prostate length (mm) | 46 | 6.4 | 24–56 | 43 | 47 | 5.8 | 37–60 | 34 |
| Prostate-specific antigen (ng/ml) | 2.4 | 1.8 | 0.4–8.2 | 43 | 2.6 | 2.1 | 0.3–8.6 | 33 |
| International Prostate Symptom Score | 22 | 5.7 | 12–33 | 44 | 23 | 5.9 | 13–34 | 35 |
| Qmax (ml/s) b | 9.2 | 3.5 | 3–15 | 39 | 9.5 | 3.2 | 3–15 | 32 |
| Post-void residual volume (ml) | 86 | 72 | 0–344 | 44 | 102 | 87 | 0–328 | 35 |
| Sexual Health Inventory for Men | 20 | 4.9 | 7–25 | 44 | 18 | 5.5 | 7–25 | 35 |
| MSHQ-EjD function | 11 | 2.7 | 4–15 | 44 | 9 | 2.3 | 4–13 | 35 |
| MSHQ-EjD bother | 1.7 | 1.8 | 0–5.0 | 44 | 2.0 | 1.5 | 0–4.0 | 35 |
| Anesthesia time (min) | 55 | 17 | 25–92 | 44 | 71 | 20 | 44–134 | 35 |
| PUL implants (n) | 4.7 | 1.1 | 2–6 | 44 | NA | |||
| Time to discharge (d) | 1.0 | 0.9 | 0–4 | 44 | 1.9 | 1.0 | 1–4 | 35 |
| Return to preoperative activity level (d) | 11 | 19 | 0–127 | 43 | 17 | 19 | 2–92 | 32 |
a PUL participant 9-003 was excluded for protocol deviation (violation of exclusion criterion).
b Qmax is not calculated for voided volume <125 ml.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; Qmax = peak flow rate; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; NA = not applicable.
PUL patients consistently had more rapid recovery than TURP patients ( Fig. 3 ). The proportion of patients achieving the BPH6 recovery endpoint by 1 mo was 82% in the PUL group, which was significantly better than the 53% in the TURP group (p = 0.008). With the original threshold, 57% PUL compared to 32% TURP patients achieved the recovery endpoint. Furthermore, 74% of the TURP group had a catheter for more than 24 h, compared to just 45% of the PUL group (p = 0.01). The average number of days to discharge was significantly lower (1.0 vs 1.9 d) and the return to preoperative activity levels was significantly faster (11 vs 17 d) for PUL than for TURP patients ( Table 3 ).

Significant improvements in IPSS, IPSS QoL, BPH II, and Qmax were observed in both arms over time ( Fig. 4 , Table 4 ). IPSS, Qmax, and PVR were better after TURP than after PUL (p < 0.05, Fig. 4 ).

Table 4 Paired outcome measures following PUL or TURP
| 2 wk | 1 mo | 3 mo | 6 mo | 12 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | PUL | TURP | |
| IPSS | ||||||||||
| n (paired) | 42 | 34 | 44 | 33 | 42 | 34 | 40 | 33 | 41 | 32 |
| BL, mean (SD) | 21.9 (5.7) | 22.6 (6.0) | 22.1 (5.7) | 22.8 (5.8) | 22.3 (5.8) | 22.6 (6.0) | 22.2 (5.7) | 22.6 (6.0) | 22.0 (5.6) | 22.8 (5.9) |
| FU, mean (SD) | 14.6 (7.7) | 15.7 (7.3) | 10.5 (7.6) | 12.9 (5.9) | 10.5 (7.4) | 10.8 (8.4) | 9.2 (7.5) | 8.0 (7.2) | 10.7 (8.1) | 7.3 (6.3) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–7.3 (9.4) –15.0, –6.5, 0.0 |
–6.8 (8.8) –14.0, –5.0, 1.0 |
–11.6 (9.3) –17.5, –12.0, –5.5 |
–10.0 (7.9) –15.0, –10.0, –4.0 |
–11.7 (8.5) –17.0, –11.0, –6.0 |
–11.8 (9.5) –20.0, –13.0, –5.0 |
–13.0 (8.1) –17.0, –13.0, –8.5 |
–14.6 (8.5) –20.0, –13.0, –9.0 |
–11.4 (8.4) –18.0, –10.0, –7.0 |
–15.4 (6.8) –20.0, –14.5, –10.5 |
| DΔ, mean (95% CI) | 0.5 (–3.7, 4.7) | 1.6 (–2.4, 5.7) | –0.1 (–4.2, 4.1) | –1.6 (–5.5, 2.3) | –4.0 (–7.7, –0.4) | |||||
| p value | 0.8 | 0.3 | 1.0 | 0.4 | 0.02 | |||||
| IPSS QoL | ||||||||||
| n (paired) | 43 | 34 | 44 | 33 | 43 | 34 | 40 | 33 | 40 | 32 |
| BL, mean (SD) | 4.7 (1.1) | 4.8 (1.2) | 4.6 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.8 (1.2) | 4.7 (1.1) | 4.7 (1.2) | 4.7 (1.0) | 4.6 (1.2) |
| FU, mean (SD) | 3.0 (1.9) | 3.7 (1.7) | 2.2 (1.8) | 3.0 (1.9) | 2.1 (1.5) | 2.4 (2.0) | 1.9 (1.6) | 1.8 (1.7) | 1.9 (1.6) | 1.5 (1.5) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.7 (2.3) –3.0, –2.0, 0.0 |
–1.0 (1.5) –2.0, –1.0, 0.0 |
–2.5 (2.0) –4.0, –2.5, –1.0 |
–1.8 (1.9) –3.0, –2.0, 0.0 |
–2.6 (1.7) –4.0, –2.0, –2.0 |
–2.4 (2.0) –4.0, –3.0, –1.0 |
–2.8 (1.6) –4.0, –3.0, –2.0 |
–2.9 (1.9) –4.0, –3.0, –2.0 |
–2.8 (1.8) –4.0, –3.0, –1.0 |
–3.1 (1.6) –4.0, –3.0, –2.0 |
| DΔ, mean (95% CI) p value |
0.7 (–0.2, 1.6) 0.1 |
0.7 (–0.2, 1.6) 0.1 |
0.3 (–0.6, 1.1) 0.5 |
–0.1 (–0.9, 0.7) 0.8 |
–0.3 (–1.1, 0.5) 0.4 |
|||||
| BPH II | ||||||||||
| n (paired) | 43 | 32 | 43 | 32 | 42 | 33 | 40 | 32 | 40 | 30 |
| BL, mean (SD) | 7.3 (2.5) | 7.2 (3.0) | 7.3 (2.5) | 7.3 (3.1) | 7.4 (2.4) | 7.3 (3.1) | 7.5 (2.4) | 7.2 (3.1) | 7.3 (2.4) | 7.0 (3.1) |
| FU, mean (SD) | 6.3 (3.3) | 7.0 (3.1) | 4.0 (3.1) | 5.3 (3.0) | 2.6 (2.8) | 3.8 (3.4) | 2.3 (2.5) | 2.2 (2.5) | 2.3 (2.8) | 1.8 (2.6) |
| Δ, mean (SD) Δ, Q1, median, Q3 |
–1.0 (4.3) –5.0, 0.0, 2.0 |
–0.2 (3.7) –3.0, 0.0, 2.5 |
–3.4 (4.3) –7.0, –3.0, –1.0 |
–2.0 (3.6) –4.5, –2.0, 1.0 |
–4.8 (3.6) –7.0, –5.0, –3.0 |
–3.4 (3.5) –5.0, –3.0, –1.0 |
–5.2 (2.9) –7.0, –5.0, –4.0 |
–5.0 (3.3) –7.0, –5.0, –3.0 |
–5.0 (3.7) –7.0, –5.0, –3.5 |
–5.2 (3.2) –6.0, –5.5, –3.0 |
| DΔ, mean (95% CI) p value |
0.8 (–1.1, 2.7) 0.3 |
1.4 (–0.5, 3.3) 0.06 |
1.4 (–0.3, 3.0) 0.05 |
0.2 (–1.3, 1.7) 0.7 |
–0.2 (–1.9, 1.5) 0.8 |
|||||
| Q max | ||||||||||
| n (paired) | 33 | 21 | 33 | 27 | 32 | 29 | ||||
| BL, mean (SD) | 9.4 (3.5) | 9.2 (3.2) | 9.6 (3.4) | 9.4 (3.2) | 9.6 (3.5) | 9.5 (3.3) | ||||
| FU, mean (SD) | 13.6 (5.3) | 22.6 (9.0) | 13.5 (5.5) | 19.0 (8.8) | 13.6 (5.5) | 23.2 (10.5) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
4.2 (5.0), 1.0, 4.0, 7.0 |
13.4 (9.9) 6.0, 11.0, 17.0 |
3.8 (5.2) 2.0, 3.0, 6.0 |
9.6 (9.2) 2.0, 8.0, 19.0 |
4.0 (4.8) 0.5, 3.0, 7.5 |
13.7 (10.4) 6.0, 11.0, 18.0 |
||||
| DΔ, mean (95% CI) p value |
9.2 (5.1, 13.3) <0.0001 |
5.8 (2.0, 9.6) 0.003 |
9.7 (5.6, 13.7) <0.0001 |
|||||||
| PVR | ||||||||||
| n (paired) | 39 | 32 | 40 | 31 | 41 | 32 | ||||
| BL, mean (SD) | 87.6 (74.1) | 98.6 (84.9) | 85.5 (73.4) | 100.5 (85.7) | 86.3 (73.2) | 103.5 (89.7) | ||||
| FU, mean (SD) | 77.3 (74.4) | 47.6 (48.7) | 80.7 (91.0) | 46.2 (49.1) | 93.7 (156.5) | 33.6 (38.6) | ||||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–10.3 (56.2) –43.0, –3.0, 21.0 |
–51.0 (78.7) –96.0, –40.5, 4.0 |
–4.8 (70.7) –33.5, –1.0, 26.5 |
–54.2 (84.6) –121.0, –34.0, 0.0 |
7.4 (115.2) –28.0, 2.0, 20.0 |
–70.0 (79.0) –121.5, –52.0, –10.0 |
||||
| DΔ, mean (95% CI) p value |
–40.6 (–72.6, –8.6) 0.002 |
–49.4 (–86.2, –12.6) 0.003 |
–77.4 (–124.9, –29.8) 0.002 |
|||||||
| SHIM | ||||||||||
| n (paired) | 36 | 20 | 38 | 27 | 34 | 30 | 32 | 27 | ||
| BL, mean (SD) | 20.3 (4.3) | 17.6 (6.2) | 20.4 (4.0) | 19.2 (5.0) | 20.6 (4.1) | 18.4 (5.4) | 20.8 (4.0) | 18.6 (5.4) | ||
| FU, mean (SD) | 20.9 (4.3) | 17.2 (7.3) | 19.7 (5.6) | 18.2 (6.5) | 20.0 (5.4) | 17.6 (6.5) | 20.7 (5.2) | 17.7 (6.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
0.6 (2.5) –0.5, 0.5, 1.5 |
–0.4 (4.9) –2.0, 0.0, 3.0 |
–0.7 (5.2) –2.0, 0.0, 1.0 |
–1.0 (5.0) –3.0, 0.0, 3.0 |
–0.5 (4.4) –1.0, 0.0, 2.0 |
–0.8 (4.6) –2.0, –0.5, 2.0 |
–0.1 (4.7) –1.0, 0.0, 2.5 |
–0.9 (4.3) –3.0, 0.0, 2.0 |
||
| DΔ, mean (95% CI) p value |
–1.0 (–3.0, 1.0) 0.3 |
–0.2 (–2.8, 2.3) 0.9 |
–0.2 (–2.5, 2.0) 0.8 |
–0.8 (–3.2, 1.5) 0.5 |
||||||
| MSHQ-EjD function | ||||||||||
| n (paired) | 36 | 18 | 38 | 27 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 10.6 (2.6) | 8.6 (2.5) | 10.8 (2.7) | 9.3 (2.4) | 10.8 (2.7) | 8.9 (2.4) | 10.6 (2.7) | 9.3 (2.1) | ||
| FU, mean (SD) | 12.3 (3.4) | 7.7 (5.0) | 11.5 (3.5) | 6.3 (4.5) | 11.9 (2.5) | 5.7 (4.2) | 11.9 (3.0) | 5.6 (4.0) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
1.7 (4.3) 0.5, 2.0, 4.0 |
–0.9 (5.0) –5.0, –1.0, 2.0 |
0.7 (3.9) 0.0, 2.0, 3.0 |
–3.0 (4.1) –6.0, –3.0, 0.0 |
1.1 (2.4) 0.0, 2.0, 3.0 |
–3.2 (4.5) –7.0, –3.0, 0.0 |
1.3 (3.3) 0.0, 2.0, 3.0 |
–3.7 (4.1) –7.0, –4.0, –1.0 |
||
| DΔ, mean (95% CI) p value |
–2.6 (–5.3, 0.0) 0.03 |
–3.7 (–5.7, –1.7) 0.0002 |
4.3 (–6.1, –2.6) <0.0001 |
–5.0 (–6.9, –3.1) <0.0001 |
||||||
| MSHQ-EjD bother | ||||||||||
| n (paired) | 36 | 18 | 38 | 28 | 35 | 29 | 32 | 27 | ||
| BL, mean (SD) | 1.8 (1.8) | 1.9 (1.7) | 1.7 (1.8) | 1.9 (1.5) | 1.6 (1.8) | 2.0 (1.6) | 1.7 (1.8) | 2.0 (1.5) | ||
| FU, mean (SD) | 1.0 (1.3) | 1.7 (1.3) | 1.1 (1.4) | 2.1 (1.4) | 1.5 (1.5) | 1.9 (1.5) | 1.2 (1.1) | 2.0 (1.3) | ||
| Δ, mean (SD) Δ, Q1, median, Q3 |
–0.8 (1.9) –2.0, 0.0, 0.0 |
–0.2 (2.3) –2.0, 0.0, 2.0 |
–0.7 (2.1) –2.0, 0.0, 0.0 |
0.2 (1.5) –1.0, 0.0, 1.0 |
–0.1 (1.9) –1.0, 0.0, 1.0 |
–0.1 (1.7) –1.0, 0.0, 1.0 |
–0.5 (2.2) –2.0, 0.0, 1.0 |
0.0 (1.5) –1.0, 0.0, 0.0 |
||
| DΔ, mean (95% CI) | 0.6 (–0.6, 1.7) | 0.9 (0.1, 1.8) | 0.0 (–0.9, 0.9) | 0.5 (–0.5, 1.5) | ||||||
| p value | 0.1 | 0.01 | 1.0 | 0.2 | ||||||
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; IPSS = International Prostate Symptom Score; BL = baseline; FU = follow-up; SD = standard deviation; CI = confidence interval; Δ = change from baseline to follow-up; Q1 = first quartile; Q3 = third quartile; DΔ = difference in Δ between PUL and TURP; IPSS = International Prostate Symptom Score; QoL = quality of life; BPH II = benign prostatic hyperplasia impact index; Qmax = peak flow rate; PVR = post-void residual volume; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction.
Erectile function was preserved in both PUL and TURP groups as measured by SHIM scores ( Fig. 4 , Table 4 ). Furthermore, the vast majority of participants achieved the BPH6 erectile function responder endpoint, with only one PUL (2.6%) and two TURP (6.1%) patients experiencing a consistent drop in SHIM score after the procedure.
Regarding ejaculatory function, the PUL group experienced an improvement in average ejaculatory score (MSHQ-EjD) from baseline (p = 0.03), but the TURP group suffered from a significant decline (p < 0.0001; Fig. 4 , Table 4 ). For the BPH6 ejaculatory assessment, the response for the PUL group was 100%, significantly better than the 60.6% response for the TURP group (p < 0.0001).
Continence preservation was comparable between the groups, and no patient experienced new-onset stress or sphincter incontinence. Of the participants who failed the BPH6 continence element (six PUL and eight TURP patients had ISI > 4 at any time), none (0/6, 0%) of the PUL patients reported new-onset pad use, whereas six TURP patients (6/8, 75%) reported that they required pads after TURP (superior PUL performance, p = 0.01).
The proportion of participants who met the original BPH6 primary endpoint was 34.9% for the PUL group and 8.6% for the TURP group (noninferiority p = 0.0002, superiority p = 0.006). The refined BPH6 primary endpoint was also met by 52.3% of PUL and 20.0% of TURP patients (noninferiority p < 0.0001; superiority p = 0.005; Table 5 ). At each follow-up interval, the responder rate was significantly higher for PUL than for TURP patients (p = 0.0002–0.006).
Table 5 BPH6 primary outcome: summary of response rates for the BPH6 endpoint and the six individual elements at 12 mo
| Response (%) | p value | Difference, % (95% CI) | ||
|---|---|---|---|---|
| PUL | TURP | |||
| Primary BPH6 endpoint | 52 | 20 | 0.005 | 32 (10, 51) |
| LUTS element (IPSS reduction ≥30%) | 73 | 91 | 0.05 | –18 (–36, 0.72) |
| Recovery element (VAS ≥70% at 1 mo) | 82 | 53 | 0.008 | 29 (6.0, 49) |
| Erectile function element (SHIM reduction <6) | 97 | 94 | 0.6 | 3 (–8.6, 18) |
| Ejaculatory function (MSHQ-EjD #3 not zero) | 100 | 61 | <0.0001 | 39 (23, 58) |
| Continence (ISI <5) | 85 | 75 | 0.4 | 10 (–9.0, 30) |
| Safety element (no Clavien-Dindo grade II+) | 93 | 79 | 0.1 | 14 (–2.3, 32) |
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate; LUTS = lower urinary tract symptoms; CI = confidence interval; IPSS = International Prostate Symptom Score; VAS = visual analog scale; SHIM = Sexual Health Inventory for Men; MSHQ-EjD = Male Sexual Health Questionnaire for Ejaculatory Dysfunction; ISI = Incontinence Severity Index.
An independent external clinical events committee comprising three board-certified urologists who were blinded to the enrollment arm adjudicated on adverse events. Medical history caused unblinding for 6% of events. All adverse events classified as treatment-related were assigned Clavien-Dindo grades according to predetermined definitions ( Table 6 ). Reintervention for failure to cure occurred in 6.8% (3/44) of PUL and 5.7% (2/35) of TURP patients (not significant; Table 7 ). No subject in either study arm started taking an alpha blocker or 5 alpha reductase inhibitor. PUL did not cause any adverse events that required surgical intervention or revision (0%). Two patients (6%) in the TURP group required surgical intervention for adverse events, a perioperative secondary hemorrhage that required revision and transurethral hemostasis, and an unpassable urethral stricture that required urethrotomy 3 mo after TURP. Furthermore, PUL patients experienced fewer treatment-related infections (7%) than TURP patients (14%; p = 0.46).
Table 6 Treatment-related AEs after PUL and TURP stratified by Clavien-Dindo classification grade
| AEs | PUL (n = 44) | TURP (n = 35) | p value | ||
|---|---|---|---|---|---|
| AEs | Patients, | AEs | Patients, | ||
| (n) | n (%) | (n) | n (%) | ||
| Clavien-Dindo grade 1 | |||||
| Bleeding | 17 | 17 (39) | 20 | 20 (57) | 0.1 |
| Irritative symptoms, pain, or discomfort | 34 | 23 (52) | 39 | 21 (60) | 0.5 |
| Urinary incontinence | 1 | 1 (2) | 6 | 6 (17) | 0.04 |
| Urinary retention | 4 | 4 (9) | 0 | 0 (0) | 0.1 |
| Erectile dysfunction | 0 | 0 (0) | 3 | 3 (9) | 0.08 |
| Retrograde ejaculation | 0 | 0 (0) | 7 | 7 (20) | 0.002 |
| Other | 4 | 4 (9) | 4 | 3 (9) | >0.9 |
| Total | 60 | 30 (68) | 79 | 26 (74) | 0.6 |
| Clavien-Dindo grade 2 | |||||
| Urinary tract infection | 3 | 3 (7) | 3 | 2 (6) | >0.9 |
| Epididymitis | 0 | 0 (0) | 2 | 2 (6) | 0.2 |
| Total | 3 | 3 (7) | 5 | 4 (11) | 0.7 |
| Clavien-Dindo grade 3a | |||||
| Total | 0 | 0 (0) | 0 | 0 (0) | |
| Clavien-Dindo grade 3b | |||||
| Bleeding | 1 | 1 (2) | 2 | 2 (6) | 0.6 |
| Stricture (meatal, urethral, bladder neck) | 0 | 0 (0) | 1 | 1 (3) | 0.4 |
| Secondary treatment (TURP, laser, PUL, or Botox) | 3 | 3 (7) | 2 | 2 (6) | >0.9 |
| Total | 4 | 4 (9) | 5 | 5 (14) | 0.5 |
AE = adverse event; PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
Table 7 Reintervention (n = 8) following treatment with PUL (n = 44) or TURP (n = 35) at early (≤30 d) and intermediate (>30–365 d) follow-up
| Adverse event | Intervention | PUL | TURP | p value a |
|---|---|---|---|---|
| Early (≤30 d) | ||||
| Bleeding (n) | Surgical revision | 0 | 1 | |
| Bleeding/bladder tamponade (n) | Evacuation | 0 | 1 | |
| Total, n (%) | 0 (0) | 2 (6) | 0.2 | |
| Delayed (>30–365 d) | ||||
| Urethral stricture | Urethrotomy | 0 | 1 | |
| Return of LUTS/dissatisfaction | Secondary treatment | 3 | 2 | |
| Total, n (%) | 3 (7) | 3 (9) | >0.9 | |
| Total early + delayed, n (%) | 3 (7) | 5 (14) | 0.5 | |
a Fisher's exact test.
PUL = prostatic urethral lift; TURP = transurethral resection of the prostate.
The BPH6 study is the first prospective, randomized trial comparing PUL with TURP. The PUL procedure not only met the primary study endpoint of noninferiority but also demonstrated superiority compared to TURP with regard to the BPH6 primary endpoint. Analysis of BPH6 element endpoints demonstrated that TURP was superior in reducing IPSS (p = 0.05), whereas PUL was superior for quality of recovery (p = 0.008) and preservation of ejaculatory function (p < 0.0001). No significant differences were observed for erectile dysfunction, incontinence, or grade II+ adverse events; this may be a result of insufficient study power for detection of differences in these elements of the BPH6.
Both study procedures effectively mitigated LUTS. At 12 mo, PUL yielded an average decrease of 11.4 ± 8.4 in IPSS, consistent with previous studies [8], [9], [10], [11], [12], [13], [14], and [15]. The IPSS improvement after TURP (15.4 ± 6.8) was also as predicted [7] and [27]. These results, among others, indicate that both PUL and TURP were performed using acceptable techniques.
One objective of a less invasive procedure is to improve surgical recovery. The recovery period after TURP can last from weeks to months, and may be disruptive for patients and their families [28] . To the best of our knowledge, this is the first study to quantify recovery experience after TURP on a visual analog scale, and it gives a powerful indication of patient experiences. The number of participants who experienced the BPH6 definition of high-quality recovery (VAS ≥70% by 1 mo) was greater for PUL than for TURP (82% vs 53%, p = 0.008). Figure 3 gives a temporal view of recovery after PUL and TURP. It is evident that TURP patients required 6–12 mo to recover to the level reached by PUL patients by 3 mo.
Iatrogenic sexual dysfunction plays a role in BPH treatment, whether medical or surgical. Clinical study results have varied widely owing to myriad instruments and reporting schemes. In this study, erectile function was largely preserved in both treatment arms, as evidenced by the stability of SHIM scores and the BPH6 erectile function element. PUL patients had a superior experience compared to TURP patients with respect to ejaculatory function: both the MSHQ-EjD scores and the BPH6 ejaculatory function element were significantly greater for the PUL group. The sexual function SHIM and MSHQ results for the PUL group are consistent with previous studies [29] and [30]. The GOLIATH study reported higher rates of de novo retrograde ejaculation in TURP patients (84/133 = 63%) [27] . The lower than expected rate of retrograde ejaculation for TURP patients in this study may be because of differences in assessment technique.
The occurrence of de novo urge incontinence after BPH therapy is not well characterized in the literature. Fewer PUL than TURP patients crossed the BPH6 incontinence threshold, but the difference was not statistically significant. Interestingly, among those who failed BPH6 for incontinence, the majority of the TURP patients (6/8, 75%) required pads, compared to 0% of the PUL patients.
Although the results for the BPH6 safety element were better for the PUL than for the TURP group, the difference was not significant. Infections treated with medication occurred more often after TURP, although the difference was not significant. Failure to cure leading to secondary treatment was considered to be a grade II+ adverse event by the clinical events committee, and occurred at a comparable rate in both arms. There were no related adverse events in the PUL group that required surgical intervention; urethrotomy and revision/transurethral hemostasis for secondary hemorrhage occurred in the TURP group.
While the study size was sufficiently powered to address the primary endpoint, it was not powered to ensure that the sample size was sufficient to detect meaningful differences in secondary endpoints. Selection bias was minimized through randomization and the development of patient enrollment materials presenting a balanced view of the procedures. Blinding was not achievable and enrollment was limited because of the nature of randomization between disparate therapies. After randomization, some subjects withdrew from the study before index treatment ( Fig. 2 ). Another limitation is the 1 yr of follow-up, as durability would be more fully assessed with data for ≥2 yr. It is important, as with all clinical studies, to apply the conclusions to the specific population studied, with a particular emphasis on sexually active men.
This study provides the first randomized comparison of PUL and TURP in men suffering from LUTS secondary to BPH. Both PUL and TURP groups achieved significant symptom relief compared to baseline, with a superior symptom relief rate for TURP. PUL was superior to TURP in terms of quality of recovery and preservation of ejaculatory function. Although designed to detect noninferiority, the study demonstrated superiority of PUL over TURP in terms of the new BPH6 responder endpoint.
Author contributions: Christian Gratzke had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sønksen, Montorsi, Chapple, Gratzke.
Acquisition of data: Sønksen, Barber, Wetterauer, Speakman, Berges, Greene, Sievert, Patterson, Fahrenkrug, Schoenthaler, Gratzke.
Analysis and interpretation of data: Sønksen, Gratzke.
Drafting of the manuscript: Sønksen, Gratzke.
Critical revision of the manuscript for important intellectual content: Chapple, Sievert.
Statistical analysis: Sønksen, Sievert.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Montorsi.
Other: None.
Financial disclosures: Christian Gratzke certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Neil J. Barber has received proctor and key opinion leader fees from NeoTract. Karl-Dietrich Sievert has received proctor and consultancy fees from NeoTract. Richard Berges, Lasse Fahrenkrug, and Martin Schoenthaler have received consultancy fees from NeoTract. Jens Sønksen, Mark J. Speakman, Ulrich Wetterauer, Damien Green, Christopher R. Chapple, Francesco Montorsi, Jacob M. Patterson, and Christian Gratzke have nothing to disclose.
Funding/Support and role of the sponsor: The study was sponsored by NeoTract Inc. The sponsor played a role in the design and conduct of the study; data management and analysis; and manuscript preparation and review.
Acknowledgments: We would like to thank the clinical research coordinators and supporters of BPH6. Special thanks to Drs. Rodney Anderson, Kyle Anderson, and Rajesh Shinghal for their work on the independent clinical events committee. We would also like to thank NeoTract Inc., especially Theodore Lamson and Jacqueline Nerney Welch, for their support in the preparation of this manuscript.