Background
Incontinence has a greater detrimental effect on quality of life than other symptoms of overactive bladder (OAB) and is often difficult to treat with antimuscarinic monotherapy.
Objective
To evaluate the efficacy and the safety and tolerability of combination (solifenacin 5 mg and mirabegron 50 mg) versus solifenacin 5 or 10 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg.
Design, setting, and participants
OAB patients remaining incontinent despite daily solifenacin 5 mg during 4-wk single-blind run-in were randomised 1:1:1 to double-blind daily combination or solifenacin 5 or 10 mg for 12 wk. Patients receiving the combination were initiated on mirabegron 25 mg increasing to 50 mg after week 4.
Outcome measurements and statistical analysis
The primary end point was a change from baseline to end of treatment (EOT) in the mean number of incontinence episodes per 24 h (stratified rank analysis of covariance [ANCOVA]). Key secondary end points were a change from baseline to EOT in the mean number of micturitions per 24 h (ANCOVA) and number of incontinence episodes noted in a 3-d diary at EOT (mixed-effects Poisson regression). A trial (BESIDE) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of combination versus solifenacin 5 mg, noninferiority (and potential superiority) of combination versus solifenacin 10 mg (key secondary end points), and the safety and tolerability of combination therapy versus solifenacin monotherapy.
Results and limitations
A total of 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719). At EOT, combination was superior to solifenacin 5 mg, with significant improvements in daily incontinence (p = 0.001), daily micturitions (p < 0.001), and incontinence noted in a 3-d diary (p = 0.014). Combination was noninferior to solifenacin 10 mg for key secondary end points and superior to solifenacin 10 mg for improving daily micturitions. All treatments were well tolerated.
Conclusions
Adding mirabegron 50 mg to solifenacin 5 mg further improved OAB symptoms versus solifenacin 5 or 10 mg, and it was well tolerated in OAB patients remaining incontinent after initial solifenacin 5 mg.
Patient summary
In this 12-wk study, overactive bladder patients who remained incontinent despite initial solifenacin 5 mg treatment received additional treatment with mirabegron 50 mg. Combining mirabegron 50 mg with solifenacin 5 mg was superior to solifenacin 5 mg alone in improving symptoms of incontinence and frequent urination, and it was well tolerated.
Trial registration
ClinicalTrials.gov NCT01908829.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).
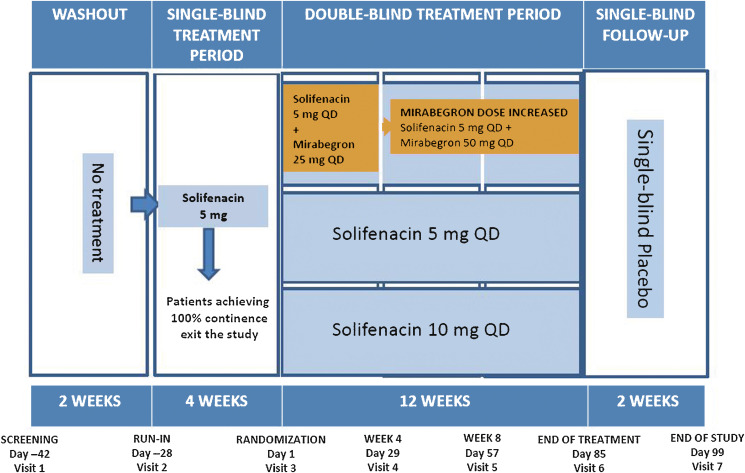
Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).
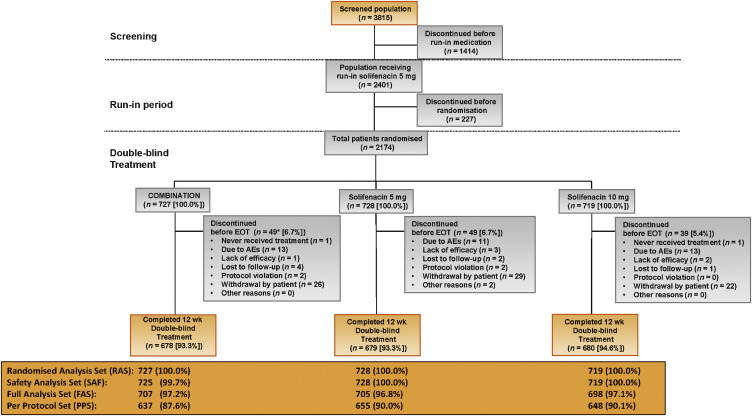
Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).
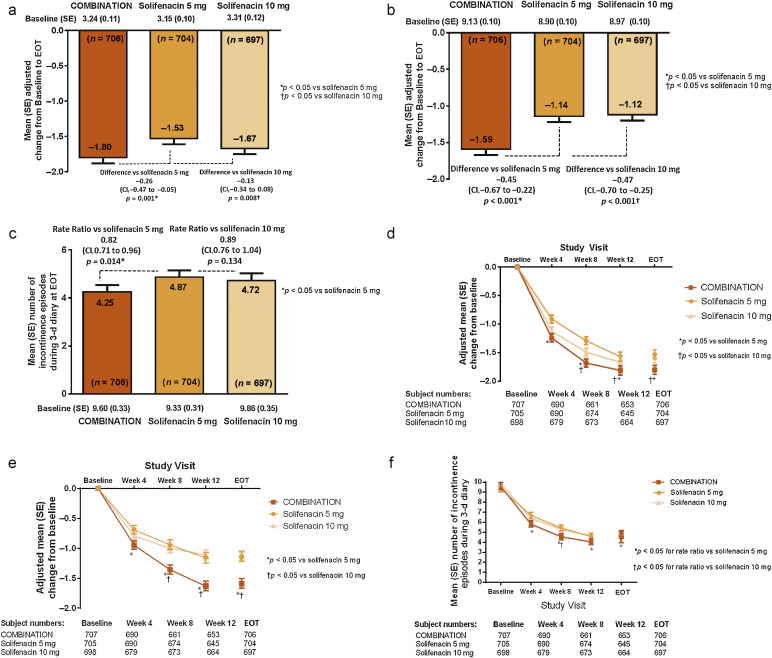
Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.
Overactive bladder (OAB) syndrome is a symptom complex defined as urinary urgency, usually accompanied by increased daytime frequency and nocturia, with or without urgency incontinence, in the absence of urinary tract infection or other obvious pathology [1] and [2]. Urgency incontinence is present in approximately one-third of OAB cases [3]. Compared with other OAB symptoms, it has the greatest impact on quality of life (QoL), with higher rates reported for depression, psychological and emotional distress, and social isolation [4]. Incontinence is associated with significantly higher health care resource utilisation and lower productivity [5]; consequently, incontinence has a major socioeconomic impact.
Oral pharmacotherapies consist of antimuscarinics (eg, solifenacin) and mirabegron, the β3-adrenoceptor agonist. Both classes of drugs share similar efficacy, but mirabegron is not associated with anticholinergic adverse events (AEs; eg, the incidence of dry mouth is comparable with placebo) [6]. In current clinical practice, patients are often initiated on antimuscarinics; however, symptom improvement is often insufficient [7], leading to dissatisfaction, particularly if incontinence persists. Increasing the antimuscarinic dose often exacerbates anticholinergic AEs that can lead to treatment discontinuation [7] and [8]. If oral therapy fails, intravesical onabotulinumtoxinA can be used to treat OAB symptoms [9] and [10], but it is associated with urinary tract infections, fluctuating response, and may require intermittent self-catheterisation [11]. Other invasive alternatives include percutaneous tibial nerve stimulation and sacral nerve stimulation [12] and [13], but their penetrance in clinical practice is limited.
A trial (BESIDE, NCT01908829) comparing combination treatment (solifenacin plus mirabegron) with one treatment alone (solifenacin) tested the superiority of a 12-wk combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4) versus solifenacin 5 mg in OAB patients remaining incontinent after 4 wk of solifenacin 5 mg. The primary objective was to evaluate the efficacy of combination versus solifenacin 5 mg. Secondary objectives were to evaluate the safety/tolerability of combination versus solifenacin 5 or 10 mg, and the noninferiority of combination versus solifenacin 10 mg. Initial experience with the combination, based on the results from an open-label postmarketing Japanese study, suggest good efficacy and tolerability with add-on mirabegron 25 or 50 mg to solifenacin 2.5 or 5 mg compared with solifenacin monotherapy [14].
In this randomised double-blind parallel-group multicentre phase 3B study, patients aged ≥18 yr with OAB symptoms for ≥3 mo, including an average of two or more incontinence episodes per 24 h, entered a 2-wk screening/washout period (visit 1) to remove the effects of previous OAB medication and familiarise themselves with the electronic micturition diary. After 4 wk of single-blind daily solifenacin 5 mg, patients remaining incontinent at baseline (one or more episodes during the 3-d diary), were eligible for double-blind treatment (Fig. 1).

Patients who satisfied inclusion and did not meet exclusion criteria (Supplementary Table 1) were randomised 1:1:1 to 12 wk of daily double-blind treatment with combination (solifenacin 5 mg and mirabegron 25 mg increasing to 50 mg after week 4), solifenacin 5 mg, or solifenacin 10 mg (Supplement 1).
During the double-blind period, efficacy was assessed using a 3-d diary prior to each study visit. The primary efficacy end point was change from baseline to end of treatment (EOT) in mean number of incontinence episodes per 24 h. Key secondary efficacy end points were change from baseline to EOT in mean number of micturitions per 24 h and the number of incontinence episodes noted in the 3-d diary at EOT. In the full analysis set (FAS; randomised patients who received one or more doses of double-blind treatment, one or more micturitions at baseline and after baseline, and one or more incontinence episodes at baseline), the primary comparison was combination versus solifenacin 5 mg; combination versus solifenacin 10 mg was a secondary analysis. A noninferiority comparison between combination and solifenacin 10 mg was performed for the key secondary end points in the per protocol set (PPS; FAS patients without major protocol violations). If noninferiority was demonstrated, the superiority of combination versus solifenacin 10 mg would be investigated.
Other secondary end points included change from baseline to weeks 4, 8, 12, and EOT in the mean number of urgency episodes (grade 3/4 on the Patient Perception of Intensity of Urgency Scale per 24 h [15]) mean volume voided micturition, mean number of urgency incontinence episodes per 24 h, mean number of pads per 24 h, mean number of nocturia episodes, Patient Perception of Bladder Condition score [16], and the percentage of patients (“responders”) achieving zero incontinence episodes at EOT. Primary and key secondary end points were also assessed at weeks 4, 8, and 12.
Safety assessments in the safety analysis set (randomised patients who received one or more doses of double-blind treatment) at each study visit and during the 2-wk single-blind placebo follow-up included the frequency of treatment-emergent AEs (TEAEs) and TEAEs of special interest (eg, antimuscarinic related), change from baseline in vital signs (systolic blood pressure [SBP], diastolic blood pressure, and pulse rate), 12-lead electrocardiogram (ECG), and postvoid residual (PVR) volume (assessed by bladder scan). Laboratory assessments were collected at screening, baseline, and EOT.
Sample size was based on previous studies with mirabegron alone and in combination with solifenacin [17], [18], [19], and [20]. A total of 610 patients per group provided 80% power to analyse incontinence based on a (nonparametric) Wilcoxon rank sum test based on ordered categories derived from the previous studies, and provided 90% power to detect a 20% reduction in 3-d incontinence episodes; 614 patients per group provided 90% power to detect a 0.50 reduction in daily micturitions for combination versus solifenacin 5 mg. Assuming a 15% dropout rate during the double-blind period, 724 patients were to be randomised to each group (Supplement 2).
Demographic and baseline OAB characteristics and all efficacy analyses were described in the FAS, except noninferiority comparisons, which were analysed in the PPS, in accordance with regulatory guidance. Last observation carried forward was used for patients who discontinued before week 12.
The primary end point (change from baseline to EOT in daily incontinence episodes) was analysed using a separate stratified rank analysis of covariance (ANCOVA) model to calculate the p value for the comparison of combination versus solifenacin 5 mg. An ANCOVA model with treatment group and randomisation stratification factors including sex, age (<65, ≥65 yr), and 4-wk incontinence episode reduction group (<50%, ≥50%) as fixed factors, and mean daily incontinence at baseline as the covariate was used to calculate adjusted changes from baseline and differences between combination and solifenacin 5 mg. The first key secondary end point, mean daily micturition frequency, was analysed using an ANCOVA model with the same fixed factors and baseline micturitions as the covariate. The number of incontinence episodes noted in the 3-d diary was analysed using a mixed-effects Poisson regression model (negative binomial to accommodate for overdispersion [21]), including treatment group, randomisation stratification factors, and log of number of incontinence episodes during baseline, to derive the rate ratio of combination versus solifenacin 5 mg (Supplement 2, sect. ii and iii).
Noninferiority testing of combination versus solifenacin 10 mg for change from baseline to EOT in mean daily micturitions was performed in the same ANCOVA model with a noninferiority margin of −0.20 micturitions per 24 h; noninferiority was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the mean treatment difference was <0.20, and superiority was concluded if the upper limit for the treatment difference was <0. The number of incontinence episodes noted in the 3-d diary was analysed using the same mixed-effects Poisson regression (negative binomial) model. The noninferiority margin was set to 1.11; noninferiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.11; superiority was concluded if the upper limit of the two-sided 95% CI for the rate ratio was <1.
Other secondary efficacy variables that were normally distributed were analysed using the ANCOVA model described for micturition frequency. Odds ratios, 95% CIs, and p values for responder rates for zero incontinence episodes at EOT were derived from a logistic regression model. Changes in vital signs were analysed using an ANCOVA model including the baseline vital sign values as covariate (Supplement 2, sect. iv and v).
Overall, 2174 patients were randomised to combination (n = 727), solifenacin 5 mg (n = 728), or solifenacin 10 mg (n = 719) (Fig. 2). Patient demographics and baseline characteristics were similar across the treatment groups (Table 1).

Table 1 Summary of demographics, baseline characteristics, and baseline characteristics related to overactive bladder (full analysis set)
| Combination n = 707 |
Solifenacin 5 mg n = 705 |
Solifenacin 10 mg n = 698 |
|
|---|---|---|---|
| Sex, n (%) | |||
| Female | 588 (83.2) | 584 (82.8) | 585 (83.8) |
| Male | 119 (16.8) | 121 (17.2) | 113 (16.2) |
| Race, n (%) | |||
| White | 671 (94.9) | 656 (93.0) | 661 (94.7) |
| Black/African American | 19 (2.7) | 24 (3.4) | 26 (3.7) |
| Asian | 13 (1.8) | 21 (3.0) | 9 (1.3) |
| Other | 4 (0.6) | 4 (0.6) | 2 (0.3) |
| Age, yr, mean (SD) | 58.0 (13.2) | 56.9 (13.4) | 57.3 (13.2) |
| ≥65 yr, n (%) | 223 (31.5) | 214 (30.4) | 214 (30.7) |
| ≥75 yr, n (%) | 71 (10.0) | 64 (9.1) | 53 (7.6) |
| BMI, kg/m2 | |||
| Mean (SD) | 29.0 (5.9) | 29.1 (6.3) | 29.0 (6.0) |
| Mean duration of OAB, mo | 75.8 | 67.8 | 70.1 |
| Previous OAB medication prior to screening, n (%) | 474 (67.0) | 487 (69.1) | 479 (68.6) |
| Previous OAB medications, n (%) | |||
| 0 | 233 (33.0) | 218 (30.9) | 219 (31.4) |
| 1 | 266 (37.6) | 268 (38.0) | 259 (37.1) |
| 2 | 114 (16.1) | 129 (18.3) | 116 (16.6) |
| >2 | 94 (13.3) | 90 (12.8) | 104 (14.9) |
| Previous OAB medication discontinued, n (%) | |||
| Insufficient effect | 423 (89.2) | 428 (87.9) | 417 (87.1) |
| Poor tolerability | 89 (18.8) | 96 (19.7) | 106 (22.1) |
| Previous solifenacin treatment prior to screening, n (%) | 269 (38.0) | 297 (42.1) | 281 (40.3) |
| Previous mirabegron treatment prior to screening, n (%) | 43 (6.1) | 39 (5.5) | 41 (5.9) |
| No. of incontinence episodes during 3-d diary, mean (SD) | 9.6 (8.9) | 9.4 (8.1) | 9.9 (9.1) |
| No. of incontinence episodes/24 h, mean (SD) | 3.23 (3.00) | 3.16 (2.73) | 3.31 (3.05) |
| No. of micturitions/24 h, mean (SD) | 9.12 (2.79) | 8.90 (2.72) | 8.96 (2.75) |
| Urgency incontinence episodes/24 h, mean (SD), n | 2.94 (2.77), 692 | 2.86 (2.49), 684 | 3.01 (2.75), 667 |
| Pads/24 h, mean (SD), n | 2.74 (2.51), 511 | 2.79 (2.38), 477 | 2.92 (2.62), 487 |
| Urgency episodes (grade 3 or 4)/24 h, mean (SD), n | 5.83 (3.83), 700 | 5.69 (3.59), 695 | 5.79 (3.72), 681 |
| Nocturia episodes/24 h, mean (SD), n | 1.51 (1.06), 538 | 1.45 (0.96), 524 | 1.50 (1.03), 532 |
BMI = body mass index; OAB = overactive bladder; SD = standard deviation.
The full analysis set included all randomised patients who took at least one dose of the double-blind study drug after randomisation and reported at least one micturition and at least one incontinence episode in the baseline diary and at least one micturition after baseline.
In the FAS, the adjusted change from baseline to EOT in the mean number of incontinence episodes per 24 h (primary end point) was statistically significantly greater with combination (−1.80) versus solifenacin 5 mg (−1.53) (Fig. 3a). At EOT, reductions in mean daily micturitions and in 3-d incontinence episodes were statistically significantly greater with combination versus solifenacin 5 mg (Fig. 3b and 3c). Combination was noninferior to solifenacin 10 mg for both key secondary end points and superior to solifenacin 10 mg for the reduction in micturition frequency using both the FAS (Fig. 3b and 3c) and PPS (Supplementary Fig. 1). Significant differences in favour of the combination were evident as early as week 4 versus solifenacin 5 mg and week 8 versus solifenacin 10 mg (Fig. 3d–f). Significantly more patients became dry at EOT with combination (46.0%) versus solifenacin 5 mg (37.9%) and 10 mg (40.2%); the odds ratios versus solifenacin 5 and 10 mg were 1.47 (95% CI, 1.17–1.84) and 1.28 (95% CI, 1.02–1.61), respectively (Table 2).

Table 2 Change from baseline to end of treatment and treatment difference versus solifenacin for the other secondary end points (full analysis set)
| Combination (n = 707) |
Solifenacin 5 mg (n = 705) |
Solifenacin 10 mg (n = 698) |
|
|---|---|---|---|
| Mean volume voided/micturition | n = 680 | n = 682 | n = 682 |
| Adjusted change from baseline to EOT, mean (SE) | 28.05 (1.97) | 16.52 (1.97) | 20.30 (1.97) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | 11.52 (2.79) (6.06–16.99) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | 7.75 (2.79) (2.29–13.21) p = 0.005 |
||
| Urgency incontinence episodes/24 h | n = 691 | n = 683 | n = 666 |
| Adjusted change from baseline to EOT, mean (SE) | –1.82 (0.07) | –1.54 (0.07) | –1.63 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.27 (0.10) (–0.47 to –0.07) p = 0.003 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.19 (0.10) (–0.39 to 0.01) p = 0.014 |
||
| Urgency episodes (grade 3 and/or 4)/24 h | n = 699 | n = 694 | n = 680 |
| Adjusted change from baseline to EOT, mean (SE) | –2.95 (0.10) | –2.41 (0.10) | –2.54 (0.11) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.54 (0.15) (–0.83 to –0.25) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.40 (0.15) (–0.69 to –0.11) p = 0.007 |
||
| Mean number of pads/24 h | n = 510 | n = 476 | n = 487 |
| Adjusted change from baseline to EOT, mean (SE) | –1.66 (0.07) | –1.35 (0.07) | –1.43 (0.07) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.31 (0.10) (–0.51 to –0.12) p = 0.002 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.23 (0.10) (–0.42 to –0.04) p = 0.020 |
||
| Mean number of nocturia episodes | n = 537 | n = 523 | n = 531 |
| Adjusted change from baseline to EOT, mean (SE) | –0.43 (0.03) | –0.37 (0.03) | –0.41 (0.03) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.06 (0.05) (–0.16 to 0.03) p = 0.174 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.02 (0.05) (–0.11 to 0.07) p = 0.634 |
||
| Change from baseline in PPBC score | n = 687 | n = 685 | n = 677 |
| Adjusted change from baseline to EOT, mean (SE) | –1.5 (0.0) | –1.2 (0.0) | –1.3 (0.0) |
| Differences of adjusted means: combination vs solifenacin 5 mg, mean (SE) (95% CI) | –0.3 (0.1) (–0.4 to –0.1) p < 0.001 |
||
| Differences of adjusted means: combination vs solifenacin 10 mg, mean (SE) (95% CI) | –0.2 (0.1) (–0.3 to –0.1) p = 0.004 |
||
| Responders for zero incontinence | |||
| Responders (%) at EOT | 325/706 (46.0) | 267/704 (37.9) | 280/697 (40.2) |
| Difference (95% CI) vs solifenacin 5 mg | 8.11 (2.97–13.24) | ||
| Odds ratio (95% CI) vs solifenacin 5 mg | 1.47 (1.17–1.84) p = 0.001 |
||
| Difference (95% CI) vs solifenacin 10 mg | 5.86 (0.69–11.04) | ||
| Odds ratio (95% CI) vs solifenacin 10 mg | 1.28 (1.02–1.61) p = 0.033 |
||
CI = confidence interval; EOT = end of treatment; FAS = full analysis set; PPBC = Patient Perception of Bladder Condition; SE = standard error.
Significant improvements in all secondary efficacy end points (except nocturia) were demonstrated with combination versus solifenacin 5 mg and for most of the end points versus solifenacin 10 mg (Table 2). Subgroup analyses showed improvements in the primary and key secondary end points with combination versus solifenacin monotherapy that were independent of age (<65 or ≥65 yr) for incontinence and micturition frequency and independent of sex for micturition frequency; improvements in incontinence with combination versus solifenacin monotherapy were evident only in the larger female population (Supplementary Table 2).
The incidence of TEAEs was lowest with solifenacin 5 mg (33.1%), highest with solifenacin 10 mg (39.4%), and 35.9% with combination; dry mouth and constipation were the most common TEAEs. Incidence of dry mouth was lower with combination (5.9%) versus solifenacin 10 mg (9.5%) and similar to solifenacin 5 mg (5.6%) (Table 3). Other differences in TEAEs included hypersensitivity reactions (combination [1.5%], solifenacin 5 and 10 mg [0.8%]) and constipation (combination [4.6%], solifenacin 5 mg [3.0%], and 10 mg [4.7%]). There were no occurrences of acute urinary retention requiring catheterisation.
Table 3 Incidence and frequency of treatment-emergent adverse events (safety analysis set)
| TEAE, n (%) | Combination (n = 725) |
Solifenacin 5 mg (n = 728) |
Solifenacin 10 mg (n = 719) |
|---|---|---|---|
| Any TEAE | 260 (35.9) | 241 (33.1) | 283 (39.4) |
| Any drug-related* TEAE | 141 (19.4) | 125 (17.2) | 161 (22.4) |
| Any serious TEAE | 13 (1.8) | 10 (1.4) | 15 (2.1) |
| Any TEAE leading to withdrawal | 11 (1.5) | 11 (1.5) | 11 (1.5) |
| Any TEAE leading to death | |||
| Common TEAEs** occurring in ≥1.5% of patients in any treatment group, n (%) | |||
| Dry mouth | 43 (5.9) | 41 (5.6) | 68 (9.5) |
| Constipation | 33 (4.6) | 22 (3.0) | 34 (4.7) |
| Oedema peripheral | 6 (0.8) | 16 (2.2) | 2 (0.3) |
| Diarrhoea | 12 (1.7) | 5 (0.7) | 6 (0.8) |
| Nasopharyngitis | 14 (1.9) | 13 (1.8) | 14 (1.9) |
| Urinary tract infection | 7 (1.0) | 7 (1.0) | 12 (1.7) |
| Headache | 9 (1.2) | 13 (1.8) | 12 (1.7) |
| Dizziness | 6 (0.8) | 11 (1.5) | 8 (1.1) |
| Somnolence | 11 (1.5) | 7 (1.0) | 4 (0.6) |
| TEAEs of interest** | |||
| Increased blood pressure | 12 (1.7) | 6 (0.8) | 13 (1.8) |
| QT prolongation | 1 (0.1) | 1 (0.1) | 2 (0.3) |
| Increased heart rate, tachycardia, atrial fibrillation, and palpitations | 7 (1.0) | 5 (0.7) | 4 (0.6) |
| Tachycardia | 2 (0.3) | 3 (0.4) | 1 (0.1) |
| Urinary tract infection | 17 (2.3) | 16 (2.2) | 20 (2.8) |
| Urinary retention† | 2 (0.3) | 1 (0.1) | 5 (0.7) |
| Acute urinary retention† | |||
| Hypersensitivity reactions | 11 (1.5) | 6 (0.8) | 6 (0.8) |
| Glaucoma | |||
* Possible or probable, as assessed by the investigator, or records where the relationship was missing.
** Based on Standardised MedDRA Queries (SMQs) or sponsor-defined lists of Preferred Terms if no SMQ was available.
† Urinary retention by preferred term, and acute urinary retention by lower level term. None of the cases of urinary retention required catheterisation in any treatment group.
SAF = safety analysis set; TEAE = treatment-emergent adverse event.
A general 1 mm Hg mean difference in SBP was observed between combination and solifenacin monotherapy at EOT (Supplementary Fig. 2). There were no notable differences in vital signs (Supplementary Fig. 2) including subpopulations stratified by hypertensive status and β-blocker use (Supplementary Table 3), and there were no notable changes in ECG parameters or PVR volume (Supplementary Table 4); very few patients (five or fewer per group) had potentially clinically significant changes in haematology or liver function parameters (Supplementary Table 5) across the three treatment groups.
Combining two oral pharmacotherapies with distinct modes of action and proven efficacy may improve OAB symptoms without exacerbating anticholinergic burden, possibly obviating the need for dose escalation or more invasive interventions.
In this significantly incontinent OAB population, who were predominantly female and characterised by an average of three or more episodes per 24 h, the once-daily combination (solifenacin 5 mg and mirabegron 50 mg) was associated with an improvement in key OAB symptoms and was well tolerated compared with solifenacin monotherapy. Combination therapy for 12 wk significantly reduced mean daily incontinence episodes (−0.26 episodes per 24 h), 3-d incontinence episodes (18% reduction), and daily micturition frequency (−0.45 micturitions per 24 h) versus solifenacin 5 mg. The magnitude of improvement in daily incontinence and micturition frequency compares favourably with mirabegron 50 mg versus placebo (−0.40 and −0.55) in “wet” and “dry” patients [22], suggesting an additive treatment effect with combination. These improvements with combination were associated with clinically meaningful improvements in patient-reported outcomes (PROs; data on file to be published). Combination was noninferior to solifenacin 10 mg for both key secondary end points (micturition frequency and 3-d incontinence) and superior to solifenacin 10 mg for micturition frequency, and several other secondary efficacy end points. The improvements observed with combination versus solifenacin 10 mg were statistically significant for all efficacy end points, with the exception of nocturia and 3-d incontinence.
Incontinent (“wet”) OAB patients experience greater impairment in QoL and physical functioning than those experiencing frequency and urgency without incontinence [23] and [24]. Therefore promoting the transition from a “wet” to a “dry” patient is expected to improve QoL significantly. In this study, incontinence reduction was clinically meaningful given the higher percentage of continent patients at EOT in the combination group; the odds of achieving zero incontinence were 47% and 28% higher with combination than solifenacin 5 or 10 mg, respectively. This compares favourably with the 32% increased likelihood of being “dry” reported with mirabegron 50 mg monotherapy versus placebo in the mirabegron phase 3 studies, where incontinence was less severe at baseline [22].
The absence of a significant improvement in nocturia with combination versus solifenacin 5 mg was probably due to the multifactorial pathophysiology and often unclear aetiology of nocturia in many patients [25]. Differences in the onset of action of combination versus solifenacin 5 mg (week 4) and versus solifenacin 10 mg (week 8) may have been related to the different mirabegron doses before and after week 4. In phase 3 mirabegron studies, the earliest significant improvement in daily incontinence was observed at week 4 with mirabegron 50 mg and week 8 with mirabegron 25 mg compared with placebo [26].
Combination and solifenacin treatments were well tolerated and generally comparable with the known safety profiles of mirabegron and solifenacin. The overall incidence of TEAEs with combination was lower than the 72.4% reported with mirabegron 50 mg add-on therapy to solifenacin 5 mg in an open-label postmarketing Japanese study [14]. The incidence of most TEAEs with the combination was similar or lower than solifenacin 10 mg. Dry mouth, the most frequently reported AE with antimuscarinics [27] and a common reason for treatment discontinuation [7], was lower with combination versus solifenacin 10 mg and similar to solifenacin 5 mg. The relatively low rate of dry mouth with solifenacin 10 mg was probably a consequence of the initial 4-wk treatment with solifenacin 5 mg, which may have encouraged the selection of more tolerable or adaptable patients to antimuscarinic-related AEs. Vital signs, ECG parameters, and PVR volume in the combination group showed no synergistic effects beyond those known from either monotherapy. The difference in SBP (approximately 1 mm Hg) with combination versus solifenacin monotherapy was attributed to a decrease in SBP with solifenacin monotherapy; the magnitude of the change in SBP with combination was similar to that observed in the mirabegron phase 3 studies [22]. There was no important heterogeneity in response according to hypertensive status or β-blocker use. A slightly higher incidence of hypersensitivity reactions with combination could in part be attributed to two cases involving clarithromycin and omeprazole use, both well-documented potential allergens [28] and [29], and two cases of allergic rhinitis. Remaining differences could be attributed to the combined hypersensitivity profiles of mirabegron and solifenacin [30] and [31]. Despite the limited incremental efficacy associated with combination versus solifenacin 10 mg, the improved tolerability profile compared with solifenacin 10 mg and information gathered through PRO tools (data on file) suggest that combining mirabegron and solifenacin may be a clinically acceptable alternative to dose escalation of solifenacin.
The option to include a mirabegron 50 mg monotherapy arm was not undertaken in BESIDE because it was designed to investigate potential benefits of add-on mirabegron therapy and not switch therapy, where such an inclusion would have been more appropriate. The inclusion of a mirabegron monotherapy arm would also have had an impact on the sample size and study duration. A 2-wk washout period for solifenacin 5 mg would be required before commencing treatment with mirabegron; this would not be the case for the combination arm. In a phase 3B noninferiority trial in OAB patients refractory to previous antimuscarinic therapy, mirabegron 50 mg did not demonstrate superiority versus solifenacin 5 mg [32]. The SYNERGY [NCT01972841] and SYNERGY II [NCT02045862] trials, which include mirabegron 25 and 50 mg arms, will provide additional 12-wk and 52-wk data on the efficacy and safety of the solifenacin/mirabegron combination.
Add-on therapy with mirabegron 50 mg for 12 wk provided greater improvements in OAB symptoms in incontinent OAB patients with an insufficient response to solifenacin 5 mg compared with solifenacin 5 or 10 mg monotherapy, and it was well tolerated. The combination of solifenacin and mirabegron needs to be evaluated in clinical practice as a potential alternative to the current approaches that include dose escalation with conventional antimuscarinic therapy or progression to more invasive third-line therapies such as intravesical botulinum toxin or neuromodulation.
Author contributions: Marcus J. Drake had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, Saleem, Huang, Siddiqui, Stölzel, Herholdt, MacDiarmid.
Acquisition of data: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Herholdt.
Analysis and interpretation of data: Drake, Chapple, Esen, Athanasiou, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Drafting of the manuscript: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Critical revision of the manuscript for important intellectual content: Drake, Chapple, Esen, Athanasiou, Cambronero, Mitcheson, Herschorn, MacDiarmid, Stölzel, Herholdt, Huang, Siddiqui, Saleem.
Statistical analysis: Stölzel, Herholdt.
Obtaining funding: Saleem.
Administrative, technical, or material support: Herholdt.
Supervision: Saleem.
Other (specify): None.
Financial disclosures: Marcus J. Drake certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Marcus J. Drake has received consultancy and speaker fees from Allergan, Astellas, and Ferring, and he has received research fees from Allergan, Astellas, Ferring, and Vysera. Christopher Chapple has received consultancy and speaker fees from Allergan, Astellas, Medtronic, Ono, and Lilley, and he has received research fees from Allergan, Ono, and Astellas. Ahmet A. Esen and Javier Cambronero have nothing to disclose. Stavros Athanasiou has received personal fees from Astellas. David Mitcheson has received consultancy and speaker fees from Astellas. Sender Herschorn has received grants and personal fees from Astellas, Pfizer, and Allergan. Tahir Saleem is a former Astellas employee. Moses Huang, Emad Siddiqui, Matthias Stölzel, and Claire Herholdt are employees of Astellas. Scott MacDiarmid has received consultancy and speaker fees from Astellas, Medtronic, Cogentix, and Allergan.
Funding/Support and role of the sponsor: The study was funded by Astellas and designed and analysed by Astellas in collaboration with the authors. Editorial assistance was funded by Astellas Pharma, Inc. Astellas had a role in the design and conduct of the study; management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Acknowledgments: The authors received medical writing assistance from Stuart Murray (Envision Scientific Solutions) for preparation of the initial and final drafts of the manuscript. The authors and study sponsor acknowledge the assistance of the BESIDE study investigators.