Background
The international prostate symptom score (IPSS) evaluates lower urinary tract symptoms (LUTS) in men with suspected benign prostatic hyperplasia (BPH); the total score does not differentiate between storage and voiding and is unevenly weighted (four questions [57%] on voiding, three questions [43%] on storage).
Objective
To evaluate the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and in response to treatment with tadalafil.
Design, setting, and participants
Integrated analysis of data from four placebo-controlled, 12-wk studies of tadalafil (5 mg once daily) in 1499 men with LUTS/BPH.
Outcome measurements and statistical analysis
Relationships between total IPSS and the storage and voiding subscores were assessed using graphical exploration and linear regression modelling. Linear modelling was performed for the baseline and endpoint and for changes in subscores. The optimal storage subscore to total IPSS (S:T) ratio for IPSS improvement was identified using nonparametric regression and gradient-descent optimisation.
Results and limitations
The contribution of storage and voiding subscores at baseline and endpoint was 38.8% and 61.2%, and 39.2% and 60.7%, respectively. This intuitive 40:60 storage-to-voiding ratio was similar at baseline and endpoint by treatment group and for changes in subscores, but spanned the entire range for individuals. Changes in total IPSS were greatest for a storage subscore percentage contribution to total IPSS of 42.7%. There was no statistical association between S:T ratio (≥40% vs <40%) at baseline and response to tadalafil. The main limitation was the use of unvalidated storage and voiding IPSS subscores.
Conclusions
A constant S:T ratio of 4:10 was observed at baseline and endpoint. The greatest effect on total IPSS was noted for an S:T percentage contribution of 42.7%. Tadalafil efficacy was unaffected by the level of storage dysfunction at baseline.
Patient summary
This analysis shows that for men with BPH, improvements during treatment with tadalafil apply to both storage and voiding symptoms at a constant ratio. The extent of storage dysfunction before treatment did not affect the response to treatment.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).
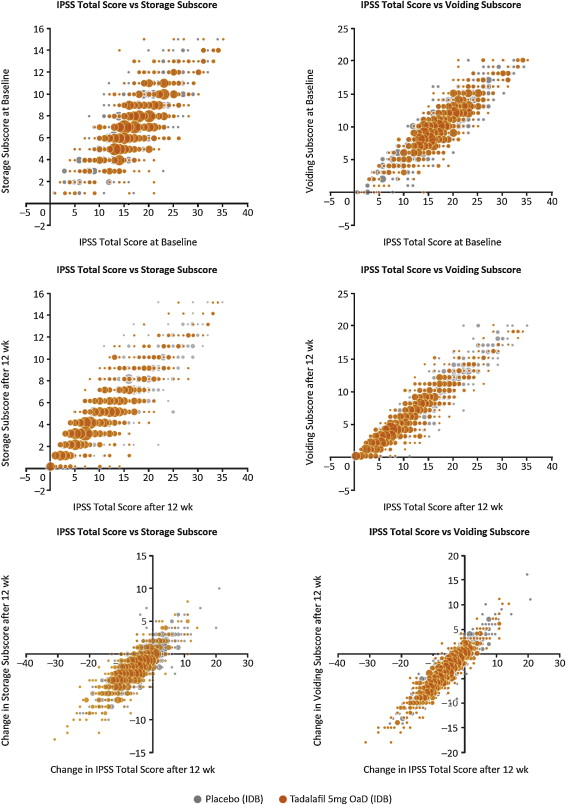
Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
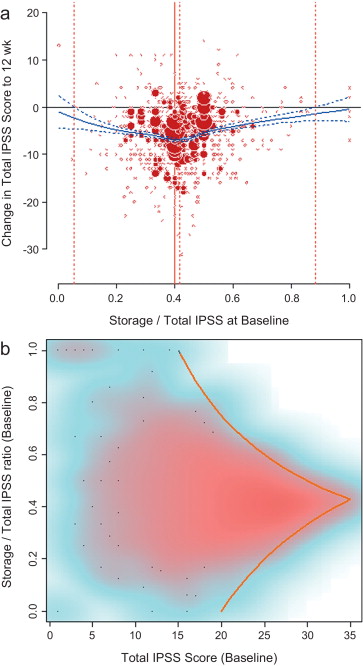
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.
There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).
Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
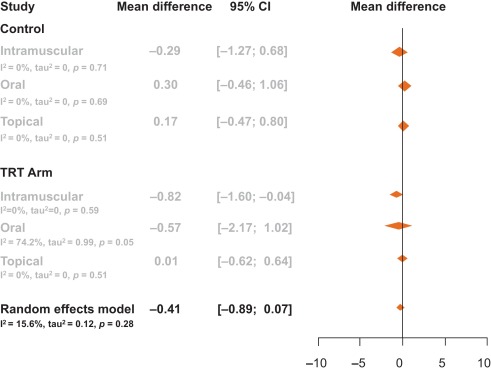
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.
In men with lower urinary tract symptoms (LUTS) who are thought to have benign prostatic hyperplasia (BPH), symptoms are commonly of a mixed nature. Storage symptoms interfere more with quality of life (QoL) than voiding symptoms do[1] and [2]and exhibit tighter correlations with QoL across all treatment modalities [3] . Storage symptoms also contribute to a greater reduction in disease-specific QoL associated with male LUTS [4] . The unpredictability of storage symptoms and their interference with activities of daily living are often a key reason for patient consultation [5] .
Treatments for LUTS/BPH traditionally target the prostate, despite the important role of the bladder in the pathogenesis of the most bothersome LUTS [6] . Storage symptoms often persist after medical therapy of LUTS/BPH[7], [8], and [9]or surgical treatment of bladder outlet obstruction [10] .
Despite its limitations, the International Prostate Symptom Score (IPSS) is internationally recognised as a validated 1-mo recall assessment of LUTS/BPH[11] and [12]. The IPSS is based on the answers to seven questions about urinary symptoms and one question concerning QoL. Each question on urinary symptoms allows the patient to choose one of six answers indicating increasing severity of the particular symptom. The answers are assigned points from 0 to 5. The total score can therefore range from 0 to 35 (1–7, mild; 8–19, moderate; 20–35, severe). A potential criticism of the IPSS is that it fails to emphasise the differential bothersomeness of storage compared with voiding symptoms. Furthermore, only one of the two typical post-micturition symptoms (feelings of incomplete bladder emptying) is assessed by the IPSS and is categorised as a voiding symptom. Despite these limitations, a reduction of ≥25% in total IPSS from baseline to endpoint has been suggested as a threshold for therapeutic response [13] , whereas a change in total IPSS of ≥3 points has been suggested as the threshold at which a patient becomes aware of potential therapeutic efficacy [11] .
Attempts have been made to determine the diagnostic value of assessing IPSS subscores[14] and [15]. Lee et al. [14] investigated the importance of improvements in storage dysfunction in Korean men with moderate to severe LUTS/BPH after combination therapy with an alpha-blocker and a 5-alpha reductase inhibitor, stratifying patients by severity of storage symptoms. However, the relative contribution of the subscore to total IPSS was not assessed. Liao et al. [15] assessed the contribution of voiding and storage subscores to total IPSS, using the voiding-to-storage subscore ratio to differentiate between voiding failure (ratio >1) and storage failure (ratio ≤1), but did not take into consideration the relative maximum contributions that voiding and storage subscores make to total IPSS (approx. 40% and 60%, respectively). The limitation of using unvalidated individual subscores has previously been highlighted [16] .
Tadalafil is a long-acting phosphodiesterase type 5 (PDE5) inhibitor approved by the US Food and Drug Administration for treatment of erectile dysfunction (ED), signs and symptoms of BPH/ED, symptoms of BPH, and pulmonary arterial hypertension (classified as World Health Organization group 1) to improve exercise ability [17] . The efficacy and safety of once-daily tadalafil in men with LUTS/BPH have been demonstrated in four pivotal, global, randomised, placebo-controlled clinical trials[18], [19], [20], and [21]. This analysis was performed to gain a better understanding of the relative effects of tadalafil on urinary storage versus voiding symptoms.
Using these four studies, we conducted an integrated analysis of baseline and outcome data for tadalafil in LUTS/BPH to assess the relative contributions of storage and voiding IPSS subscores to total IPSS at baseline and the impact of these contributions on the response to treatment in terms of total IPSS at endpoint. The varying contributions of the subscores to the total score are not often taken into consideration when making management decisions for men with LUTS, yet a preponderance of one pattern of disease (even in men with the same total IPSS) can impact the bothersomeness of disease at baseline, as well as response to therapy.
This integrated post hoc analysis used pooled data from four randomised, double-blind, placebo-controlled, 12-wk studies of once-daily tadalafil in men with LUTS/BPH[18], [19], [20], and [21]with similar inclusion and exclusion criteria. Participants were aged ≥45 yr with a history of LUTS secondary to BPH for >6 mo, IPSS ≥13, and a peak urinary flow rate of 4–15 ml/s. In all studies, a 4-wk washout of other LUTS/BPH medication preceded a 4-wk placebo lead-in period before the baseline visit. At baseline, patients were randomised to either tadalafil 5 mg once daily or placebo for 12 wk. The primary outcome was improvement in total IPSS from baseline to endpoint (12 wk).
Relationships between subscores and total scores were explored by mapping the distribution of the storage subscore to total IPSS (S:T) and voiding subscore to total IPSS (V:T) at baseline and endpoint, as well as the distribution of changes in these scores from baseline to endpoint. The results are presented as bubble plots.
Relationships were further explored using linear regression modelling, adjusting for study, to estimate the change in IPSS subscores as a fraction of the change in total IPSS (95% confidence interval [CI]) in all patients.
Bubble plots were also used to investigate relationships between S:T and total IPSS at baseline, and between S:T at baseline and the change in total IPSS from baseline to 12 wk. Local polynomial regression (LPR) modelling [22] was used to describe the relationship between change in total IPSS in response to treatment and as a function of the S:T at baseline to determine the S:T ratio corresponding to the maximum improvement in total IPSS. LPR modelling was chosen because it is a nonparametric method that makes minimal assumptions about the functional form of a relationship. The S:T ratio resulting in the maximum change in total IPSS was obtained using a numerical gradient-descent procedure. S:T ratios with no change in total IPSS (LPR + 1 × standard error [SE]) were determined using a root-finding procedure. A margin of 1 SE was applied to increase the certainty of identifying the interval of positive improvement.
Finally, patients were stratified according to the contribution of the storage subscore to total IPSS at baseline (≥40% vs <40%) and analysed according to whether they were considered as responders (≥25% improvement in total IPSS from baseline) or non-responders (<25% improvement) using the Cochran-Mantel-Haenszel test (adjusted for age and baseline severity score). SAS 9.2, R 2.15.2, and Microsoft Excel 2010 were used for the statistical analyses.
For context, simple literature searches were performed to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores for comparison. Raw arithmetic means for total IPSS and IPSS storage and voiding subscores at baseline and endpoint were extracted from the published data to allow calculation of the S:T ratio.
Of 1499 men with LUTS thought to be secondary to BPH, 752 were randomised to tadalafil and 747 to placebo[18], [19], [20], and [21].
Relationships between storage and voiding subscores and total IPSS at baseline and endpoint, and the change from baseline to endpoint, are presented in Figure 1 . Linear regression modelling suggested that the percentage contribution of the storage subscore to total IPSS was 38.8% (95% CI 37.2–40.5%) at baseline and 39.2% (95% CI 38.1–40.4%) at endpoint. The voiding subscore contribution was 61.2% (95% CI 59.6–62.8%) at baseline and 60.7% (95% CI 59.6–61.9%), at endpoint ( Table 1 ). These relationships are in line with the theoretical ratio (43:57) for the contribution of storage and voiding subscores to total IPSS according to the number of subscore questions for urinary storage (3 questions) and voiding (4 questions).

Table 1 Regression model estimates for the linear relationship between IPSS subscore and total IPSS a
| Linear regression estimate (95% CI) for total IPSS | Timepoint | Population | ||
|---|---|---|---|---|
| Storage | Voiding | |||
| Subscore | 0.388 (0.372–0.405) | 0.612 (0.596–0.628) | Baseline | ITT |
| 0.389 (0.365–0.412) | 0.611 (0.588–0.635) | Baseline | Tadalafil | |
| 0.387 (0.365–0.410) | 0.613 (0.590–0.635) | Baseline | Placebo | |
| 0.392 (0.381–0.404) | 0.607 (0.596–0.619) | 12 wk | ITT | |
| 0.393 (0.376–0.410) | 0.606 (0.589–0.623) | 12 wk | Tadalafil | |
| 0.391 (0.375–0.408) | 0.609 (0.593–0.625) | 12 wk | Placebo | |
| Change in subscore | 0.384 (0.371–0.397) | 0.616 (0.603–0.629) | Baseline to 12 wk | ITT |
| 0.380 (0.361–0.398) | 0.620 (0.602–0.639) | Baseline to 12 wk | Tadalafil | |
| 0.392 (0.373–0.411) | 0.608 (0.590–0.627) | Baseline to 12 wk | Placebo | |
| Theoretical ratio | 0.4 | 0.6 | ||
a The model included an additional adjustment for study.
CI = confidence interval; IPSS = International Prostate Symptom Score; ITT = intention to treat.
LPR modelling identified a storage subscore contribution of 42.7% to total IPSS as that at which the numerical improvement in total IPSS was largest ( Fig. 2 a). The percentage contribution of the storage subscore to total IPSS associated with an improvement in total IPSS potentially ranged from 5.4% to 88.3%. The distribution of possible S:T ratios and total IPSS combinations at baseline for the pooled tadalafil and placebo groups, and the theoretical limits of this distribution, are shown in Figure 2 b.

There was no association between S:T ratio at baseline and response to tadalafil, with no significant differences between high and low degrees of storage dysfunction at baseline (S:T ≥40% vs <40%) and response to tadalafil (improvement in IPSS from baseline to endpoint ≥25% vs <25% [no response]; odds ratio [OR] 0.94, 95% CI 0.7–1.3; Fig. 3 a).

Storage symptom subscores contributed to ≥40% of total IPSS in 63% of patients compared with 37% of patients with storage subscores of <40%; therefore, a greater proportion of patients in the responder group had a storage contribution ≥40% ( Fig. 3 b). However, there was no statistical association between response to tadalafil (change in IPSS ≥25% vs <25%) and S:T of ≥40% or <40% (OR 1.07, 95% CI 0.8–1.5).
The mechanism of action of the long-acting PDE5 inhibitor tadalafil in the treatment of men with LUTS secondary to BPH is believed to be associated with stimulation of increased activity of the nitric oxide/cGMP/protein kinase G pathway via inhibition of PDE5 isoenzymes in different tissues of the lower urinary tract. It is postulated that this results in (1) smooth muscle relaxation in the bladder, urethra, prostate, and supporting vasculature, (2) increased blood perfusion to the pelvic area, and (3) modulation of sensory stimuli from this area[23] and [24]. The integrated analysis of almost 1500 men with LUTS presented here supports the suggested beneficial impact of tadalafil on the bladder, prostate, and urethra, with largely similar reductions in storage (the most bothersome issue) and voiding symptoms.
This analysis explored the relationships between total IPSS and storage and voiding subscores of the IPSS, both before and at the end of treatment with tadalafil. These relationships have not been studied in detail before, yet are likely to have an impact on the response to treatment and hence choice of therapy. While it has been postulated that the 40:60 split in S:V subscores may be a function of the IPSS tool itself, our integrated analysis reveals that the range of S:V subscore ratios for individuals in our data set spans the entire possible spectrum ( Fig. 2 b). Importantly, across this whole spectrum, the efficacy of tadalafil is unaffected. These results suggest that symptom improvements in response to tadalafil treatment in men with BPH do not differ between high and low degrees of storage dysfunction at baseline, as illustrated in Figures 1 and 2a.
It is now well recognised that storage LUTS are the most bothersome for symptomatic patients. However, algorithms for the management of patients with predominantly storage LUTS or predominantly voiding LUTS offer generic guidance to clinicians with respect to the relative proportions of storage to voiding LUTS and their severity, and this reflects the lack of published information on this subject. This emphasises the importance of our analysis, which offers reassurance that the IPSS storage and voiding subscores maintain a tight, fixed ratio to each other. Although this could be predicted from the design of the IPSS and by bearing in mind that only three of the seven questions in the IPSS consider storage symptoms, it is important to emphasise that separate analysis of IPSS storage and voiding subscores is not validated [25] .
Previous reports have suggested that the beneficial effects of tadalafil in men with LUTS/BPH are driven by improvements in both storage and voiding elements of the IPSS. In accordance with this, our integrated analysis showed that both storage and voiding moieties of the IPSS were significantly improved in the active treatment arms compared with placebo (p < 0.001) [26] . In this integrated analysis, IPSS storage and voiding subscores made a nearly linear contribution to total IPSS in a 4:6 ratio that was maintained from baseline through to study endpoint (12 wk). These relationships are in line with the theoretical ratio (43 (3/7):57 (4/7)) for the contribution of storage and voiding subscores to total IPSS.
An S:T percentage contribution of 42.7% was associated with the largest change in total IPSS in patients receiving tadalafil ( Fig. 2 a and 2b). This was based on summation for the whole population, but individual patients displayed numerous combinations of storage and voiding subscores that gave rise to the same total IPSS, as shown in Figure 2 b. The potential range of the storage subscore contribution to total IPSS in cases for which symptomatic improvement was recorded ranged from 5.4% to 88.3%. This indicates that irrespective of the level of severity of storage symptoms at baseline, most patients receiving tadalafil were likely to experience an improvement in total IPSS by endpoint. Nevertheless, the most frequent total score for IPSS at baseline was 15, and the baseline S:T percentage contribution most commonly associated with this score was 40% ( Fig. 2 a). Again, the range of S:T ratios observed spanned almost the entire spectrum and covered all possible total IPSS results within the theoretical limits of the distribution. These results suggest that storage, as measured by the IPSS, cannot solely drive improvements in urinary symptoms.
The aim of our analysis was to assess the relative contributions of storage and voiding subscores to total IPSS at baseline, and how these contributions impact on the response to treatment in terms of total IPSS at endpoint. Although the aim was not to answer questions on the mechanism of action of tadalafil or to look at responses to individual questions in the IPSS, the study provides another important part of this complex picture. It is difficult to associate symptom improvements with theories on the mechanism of action of a drug, and it is known that the correlation between symptoms and uroflowmetry is poor. However, a recent integrated analysis of data for more than 1300 men with LUTS/BPH identified a small but significant improvement in median maximum urinary flow rate for tadalafil versus placebo [27] . In addition, the contributions of individual IPSS scores to response to tadalafil treatment have been the subject of a recently published study [28] . The results show that all IPSS scores improved somewhat more than the score for IPSS Q7 (nocturia/nocturnal voiding) in response to tadalafil treatment, and that the Q7 score appeared to make a greater relative contribution to total IPSS at endpoint than at baseline (14.6% and 12.8%, respectively). In an attempt to compare our results with currently approved first-line treatments for LUTS/BPH, we performed simple literature searches to identify studies of alpha-blockers for which baseline and outcome data were available for IPSS subscores. Few studies of alpha-blockers in the treatment of patients with LUTS/BPH have analysed the IPSS storage and voiding subscores in this way. The data compiled from placebo-controlled trials identified in our literature searches are summarised in Table 2 . Differences in study design, inclusion/exclusion criteria, and study populations mandate caution when making any comparisons and make it difficult to draw any definitive conclusions. With these caveats in mind, some noteworthy points arise from our analysis. While acknowledging the degree of variation in the percentage contribution of the storage subscore to the total score across the alpha-blockers evaluated, the range is still centred around 40%, with improvements at endpoint ranging from 30% to 48% with a mean of 37% ( Fig. 4 ). Thus, approximately 40% of the symptom improvement seen with both alpha-blockers and tadalafil in men with LUTS/BPH appears to be driven by improvements in the storage subscore. The historical explanation of the mode of action of alpha-blockers may not fully explain this finding, and it may be that this 40:60 ratio is more a function of the nature of the IPSS measurement tool than of a specific therapeutic effect of the alpha-blocker class. Nevertheless, the finding does add some weight to the contemporary view that challenges the assumption that alpha-blockers act solely via urodynamic deobstruction [38] .
Table 2 Mean change in storage and voiding IPSS subscores from baseline according to data from studies of alpha-blockers in LUTS/BPH in comparison to results from the present analysis
| Reference | S:T at baseline (%) | Baseline SIPSS | SIPSS change from baseline | Baseline VIPSS | VIPSS change from baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Placebo | Drug | Placebo | Drug | Placebo | p value | Drug | Placebo | Drug | Placebo | p value | |
| Silodosin | ||||||||||||
| [29] | 41.1 | 41.0 | 7.9 [2.49] | 8.0 [2.64] | −2.5 | −1.8 | 0.002 | 11.3 [3.13] | 11.3 [3.22] | −4.5 | −2.9 | <0.001 |
| [30] | 37.2 | 36.6 | 6.4 [3.0] | 6.3 [2.8] | −2.5 [2.9] | −1.5 [2.6] | 0.006 | 10.8 [4.1] | 10.9 [4.4] | −5.8 [4.6] | −3.8 [4.8] | <0.001 |
| [31] a | 47.2 | NR | 7.97 [3.88] | – | −1.45 | – | 8.93 [3.95] | – | −1.65 | – | ||
| [32] | 36.9 | NR | 7.1 [3.1] | – | −3.5 [2.2] | – | 12.1 [3.3] | – | −7.1 [3.8] | – | ||
| Tamsulosin | ||||||||||||
| [29] | 41.7 | 41.4 | 7.9 [2.51] | 8.0 [2.64] | −2.4 | −1.8 | 0.002 | 11.0 [3.27] | 11.3 [3.22] | −4.2 | −2.9 | <0.001 |
| [30] | 36.4 | 36.6 | 6.2 [2.9] | 6.3 [2.8] | −2.1 [2.6] | −1.5 [2.6] | 10.8 [4.2] | 10.9 [4.4] | −4.8 [4.1] | −3.8 [4.8] | ||
| [33] b | 43.0 | NR | 7.2 [2.9] | – | −1.4 | – | 9.2 [4.2] | – | −2.8 | – | ||
| [34] c | 42.1 | 41.7 | 7.8 [2.6] | 7.6 [2.6] | −3.0 [2.8] | −2.2 [2.7] | <0.001 | 10.7 [3.4] | 10.6 [3.4] | −4.7 [4.0] | −3.7 [3.8] | <0.001 |
| [32] | 34.7 | NR | 6.9 [3.1] | – | −3.3 [2.2] | – | 13.0 [3.3] | – | −6.7 [3.9] | – | ||
| Alfuzosin | ||||||||||||
| [35] | 42.0 | 41.0 | 7.9 [2.8] | 7.7 [2.5] | −2.3 [2.8] | −1.6 [2.7] | <0.001 | 10.9 [3.5] | 11.1 [3.2] | −3.8 [3.7] | −2.9 [3.9] | <0.001 |
| [36] | 43.8 | NR | 7.9 [2.9] | – | −2.8 [0.3] | – | 10.1 [3.8] | – | −4.7 [0.4] | – | ||
| [37] d | 41.7 | NR | 7.9 [4.2] | – | −2.14 | – | 11.0 [4.9] | – | −3.93 | – | ||
| Tadalafil | ||||||||||||
| Present analysis | 42.0 | 38.7 | 7.4 | 7.4 | −2.0 | −1.1 | <0.001 | 10.2 | 9.9 | −3.2 | −1.8 | <0.001 |
a Endpoint day 1.
b 6-mo data.
c Tamsulosin oral controlled absorption system 0.4 mg.
d 3-mo data.
IPSS = International Prostate Symptom Score; BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms; S:T = ratio of storage subscore to total IPSS; SIPSS = storage IPSS subscore; VIPSS = voiding IPSS subscore; NR = not recorded.
SIPSSand VIPSSdata are presented as mean [SD] except for studies for which no SD were reported.

Fig. 4 Contribution of storage symptoms to total symptoms calculated as the mean change in IPSS from baseline following treatment with an alpha-blocker or tadalafil in men with LUTS/BPH according to published data. The dashed line indicates a 40% contribution of the storage subscore to total International Prostate Symptom Score (IPSS). The contribution of the storage subscore to total IPSS was calculated using published data; total IPSS is defined as the sum of storage and voiding subscores. BPH = benign prostatic hyperplasia; LUTS = lower urinary tract symptoms.
In this analysis, the efficacy of tadalafil from baseline to study end at 12 wk did not differ between men with high and low degrees of storage dysfunction at baseline (as indicated by S:T ≥40% or <40%, respectively). Improvements in total IPSS were driven by an average 40% contribution from the storage subscore, a result in accordance with previous studies on alpha-antagonist therapy. These findings lend support to the view that contemporary pharmacotherapy for LUTS—at the therapeutic doses used in clinical practice—acts via a number of mechanisms. These are likely to include a potentially important effect on sensory and motor mechanisms involved in lower urinary tract dysfunction.
Author contributions: Christopher Chapple had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Chapple, Roerhborn, Ilo, Henneges.
Acquisition of data: Ilo.
Analysis and interpretation of data: Chapple, Roerhorn, McVary, Ilo, Henneges, Viktrup.
Drafting of the manuscript: Chapple, McVary, Ilo, Henneges, Viktrup.
Critical revision of the manuscript for important intellectual content: Chapple, Roerhorn, McVary, Henneges, Viktrup.
Statistical analysis: Ilo, Henneges.
Obtaining funding: None.
Administrative, technical, or material support: Henneges, Viktrup.
Supervision: Chapple, Roerhorn, Ilo, Henneges.
Other(specify): None.
Financial disclosures: Christopher Chapple certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Christopher Chapple is a consultant for AMS, Allergan, Astellas, Lilly, ONO, Pfizer, and Recordati, and a researcher, speaker, and trial participant for Allergan, Astellas, Pfizer, and Recordati. Claus Roehrborn has been a paid consultant to Eli Lilly regarding the development of tadalafil for male LUTS and BPH. Kevin McVary is a consultant/advisor for Allergan, Eli Lilly/ICOS, NxThera, Watson Pharmaceuticals, and Neotract, He has participated in scientific studies/trials for Allergan, Lilly/ICOS, and NxThera, has received honoraria from Allergan, Eli Lilly/ICOS, NxThera, and Watson Pharmaceuticals, has been a principal investigator for Allergan, Eli Lilly/ICOS, Neotract, and the National Institute of Diabetes and Digestive and Kidney Diseases, and participated in meetings for GlaxoSmithKline. Dapo Ilo, Carsten Henneges and Lars Viktrup are all employees of Eli Lilly.
Funding/Support and role of the sponsor: This study was supported by Eli Lilly, who played a role in the design and conduct of the study, collection of the data, management of the data, analysis, interpretation of the data, and preparation, review, and approval of the manuscript.
Acknowledgement statement: This study was supported by Eli Lilly. The authors would like to acknowledge Clare Gurton and David Peters (Rx Communications, Mold, UK) for medical writing assistance during the preparation of this article, and Helmut Petto (El Lilly Regional Operations GmbH, Austria) for his help with statistical programming and analysis.