Context
Monopolar transurethral resection of the prostate (M-TURP) is the current UK surgical standard of care for benign prostatic hyperplasia, a condition estimated to affect >2 million men in the United Kingdom. Although M-TURP efficacy in prostate resection is established, potential perioperative complications and associated costs remain a concern.
Objective
To present up-to-date and robust evidence in support of bipolar transurethral resection in saline (TURis) as an alternative surgical option to M-TURP.
Evidence acquisition
A systematic review (SR) of electronic databases (up to 2015) for randomised controlled trials (RCTs) comparing TURis with M-TURP was conducted, followed by evidence synthesis in the form of a meta-analysis of hospital stay, catheterisation time and procedure duration, transurethral resection (TUR) syndrome, blood transfusion, clot retention, and urethral strictures. An economic analysis was subsequently undertaken from the UK National Health Service hospital perspective with costs and resource use data from published sources.
Evidence synthesis
The SR identified 15 good-quality RCTs, of which 11 were used to inform the meta-analysis. TURis was associated with improved safety versus M-TURP, eliminating the risk of TUR syndrome and reducing the risk of blood transfusion and clot retention (relative risks: 0.34 and 0.43, respectively; p < 0.05). TURis also reduced hospital stay (mean difference: 0.56 d; p < 0.0001). The economic analysis indicated potential cost savings with TURis versus M-TURP of up to £204 per patient, with incremental equipment costs offset by savings from reduced hospital stay and fewer complications.
Conclusions
The TURis system is associated with significant improvements in perioperative safety compared with M-TURP while ensuring equivalent clinical outcomes of prostate resection. The safety benefits identified may translate into cost savings for UK health services.
Patient summary
Our review of bipolar transurethral resection in saline, the new prostate resection technique, indicates that it offers equal efficacy while reducing complications and length of hospital stay.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
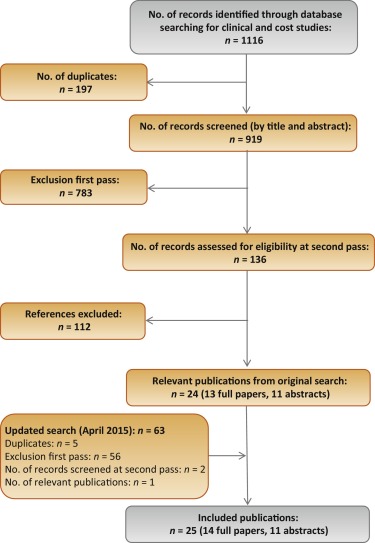
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.
Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.
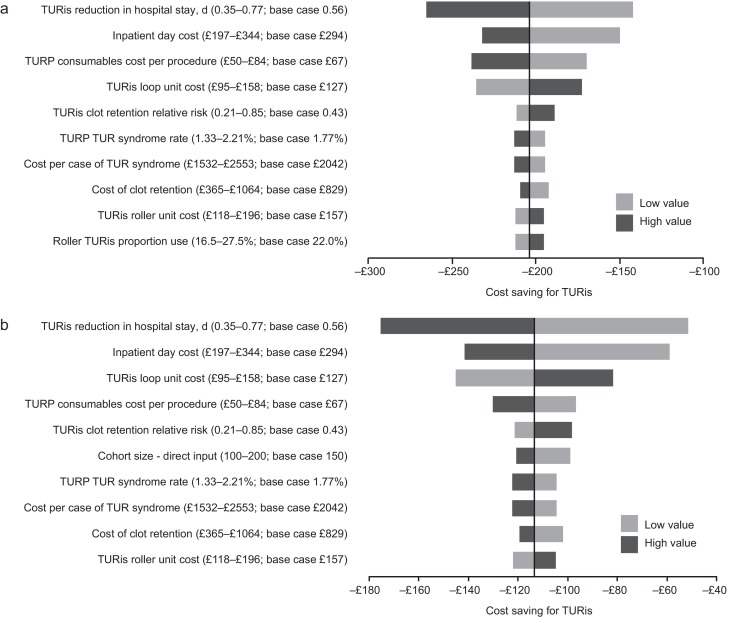
Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in men [1] including high urinary frequency, nocturia, and urgency (storage symptoms), and weak or intermittent urinary stream, incomplete bladder emptying, and postmicturition dribbling (voiding symptoms) [2] and [3]. Prevalence of LUTS increases with age; approximately one-third of men aged >65 yr experience symptoms that negatively affect daily living [3]. Patients experience quality-of-life reductions that increase with symptom severity; 45–54% of patients with moderate to severe symptoms report anxiety and/or depression [4]. Treatment of BPH and LUTS places a considerable cost burden on health care services. In 2008–2009, estimated total UK annual drug therapy cost for BPH was >£69 million. Secondary care costs of treating BPH were estimated at £112 million per annum, £55 million attributable to BPH-related surgery [5].
In men with mild/moderate LUTS, current UK and European guidelines recommend conservative management involving watchful waiting with or without behavioural and dietary modification, or medication to control symptoms [3] and [6]. Surgical intervention is offered to patients with severe voiding symptoms presumed secondary to BPH or if first-line treatment is unsuccessful or considered inappropriate [3] and [6]. Transurethral resection of the prostate (TURP) is the most commonly used surgical procedure for endoscopic removal of excess prostate tissue in the treatment of BPH [7] and is recommended for prostate volumes of 30-80 g [6]. Other surgical interventions include laser vaporization, enucleation, and open prostatectomy, restricted to patients with estimated prostate sizes >80 g [3] and [8].
The most common perioperative complications of TURP are postoperative bleeding requiring transfusion (1–3% of patients) [9] and [10], clot retention (2–5% of patients) [11], urinary tract infection (4% of patients) [10], and urethral strictures (2–10% of patients) [11]. A further potential and possibly severe complication of TURP is systemic absorption of irrigation fluid [12]. Monopolar TURP (M-TURP), the system conventionally used for surgical treatment of BPH, uses a glycine, sorbitol, or mannitol solution as a nonconducting irrigation fluid [12]. Excessive systemic absorption of this solution during the procedure can result in transurethral resection (TUR) syndrome, reported to occur in 1.4% of procedures [10]. Symptoms of TUR syndrome include headache, bradycardia, abdominal distension, nausea and vomiting, confusion, and convulsions [13]. Untreated, it can lead to pulmonary or cerebral oedema or coma [13] and [14], or death in 0.2–0.8% of cases [15].
In England and Wales, an estimated 15 000 prostate resection procedures are performed annually [16]. Over the last 10 yr, use of M-TURP for surgical treatment of BPH has been challenged by the introduction of novel procedures including bipolar technology. Bipolar electrosurgical techniques, where both active and return electrodes are contained in the resectoscope, are currently the most extensively investigated alternative to M-TURP [17]. This design means that no patient return electrode is required, enabling the use of a physiologic saline irrigation fluid. Because the saline is near isotonic with blood, the risk of TUR syndrome as a result of systemic uptake is minimised [17]. The fluid volume uptake should still be carefully monitored, especially in patients with cardiac or pulmonary conditions. A comparison of the efficacy and safety of these techniques was recently performed by Cornu et al [18]. Although it is acknowledged that bipolar TURP (B-TURP) offers a more favourable perioperative safety profile than M-TURP [6] and [19], there is currently no European consensus on its use.
The transurethral resection in saline (TURis) system uses a bipolar generator, where the energy creates a plasma corona at the electrode tip. The case for adopting TURis as a bipolar alternative to M-TURP was recently evaluated in the National Institute for Health and Care Excellence (NICE) medical technologies evaluation programme (MTEP). The evaluation resulted in publication of NICE medical technology guidance 23 (MTG23) in February 2015. It concluded that clinical and economic evidence supports the adoption of TURis for the surgical treatment of BPH [20]. The adoption of TURis is not anticipated to be associated with a steep learning curve given the similarity of the resection technique to M-TURP.
To incorporate recently published evidence for TURis versus M-TURP, we conducted an update to the original systematic review (SR), meta-analysis, and economic analysis presented in the manufacturer's MTEP submission. Preliminary results were presented at the 2015 World Congress of Urology [21].
An SR was performed to identify clinical trials to form the basis of a meta-analysis. The results of the clinical meta-analysis form one part of our objective. They were also used as input for an economic analysis, the second part of our objective.
The SR was performed in line with guidance by the Centre for Reviews and Dissemination, University of York [22]. The detailed SR methodology, as well as results and quality assessment, is reported in the supplementary information for MTG23 [20]. We performed an update to the original search on April 20, 2015, limited to randomised controlled trial (RCT) publications from 2014 onwards. The output from this update was then combined with that from the original SR (Table 1).
Table 1
Selection criteria for published and unpublished studies
| Inclusion criteria | ||
|---|---|---|
| Published studies | Unpublished studies | |
| Population | Adults with LUTS presumed secondary to BPH, for whom surgical intervention, mostly commonly TURP, is indicated Individuals with prosthetic lower limbs or cardiac pacemakers |
|
| Interventions | TURis Monopolar TURP |
|
| Outcomes | Hospital length of stay Procedural blood loss and blood transfusion requirement Time of removal of urinary catheter postoperatively TUR syndrome Readmittance for repeat procedures Duration of surgical procedure Incidence of clot retention Quality of life Device-related adverse events |
|
| Study design | Any clinical study | |
| Language restrictions | English-language full papers English abstracts of foreign-language papers |
|
| Search dates | Medline in-process and other nonindexed citations and Ovid Medline: 1946–present Embase: 1980–2014, week 9 EBM Reviews: NHS Economic Evaluation Database, first quarter 2014 EBM Reviews: Cochrane Central Register of Controlled Trials, January 2014 EBM Reviews: Cochrane Database of Systematic Reviews, 2005 to January 2014 EBM Reviews: Database of Abstracts of Reviews of Effects, first quarter 2014 EBM Reviews: Health Technology Assessment, first quarter 2014 Econlit: 1886 to February 2014 |
NA |
| Exclusion criteria | |
|---|---|
| Population | NA |
| Indication | Prostate cancer |
| Interventions | Bipolar TURP devices other than TURis Plasmakinetic vaporisation Comparator interventions other than monopolar TURP (eg, laser prostatectomy) |
| Outcomes | NA |
| Study design | NA |
| Language restrictions | Non–English language |
| Search dates | NA |
BPH = benign prostatic hyperplasia; EBM = evidence-based medicine; LUTS = lower urinary tract symptoms; NA = not applicable; NHS = National Health Service; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Expert opinion solicited during development of the MTEP submission suggested that differences are most commonly observed between TURis and M-TURP in the incidence of TUR syndrome, blood transfusions, clot retention, and hospital stay. The evidence was synthesised using pairwise meta-analysis techniques to determine any evidence-based differences between the treatments in these outcomes, as well as in other commonly reported outcomes including urethral strictures, catheterisation time, and procedure duration. According to the Cochrane Systematic Review Handbook, a minimum of 10 studies is required to draw meaningful conclusions from random-effects calculations [5]. Because each meta-analysis undertaken involved <10 studies, fixed-effects analyses were conducted. Details of the meta-analysis methodology were previously reported [20].
The economic analysis (a cost-consequence analysis) compared costs and outcomes associated with TURis and M-TURP, respectively, for surgical treatment of BPH. The perspective of the analysis was that of the National Health Service (NHS) and Personal Social Services in England and Wales.
The analysis considered adult men with LUTS presumed secondary to BPH, in whom surgical intervention was indicated.
A decision-tree model was constructed in which men with BPH-related LUTS underwent surgery with either TURis or M-TURP. Patients entered the model at the point of being indicated for surgery and were allocated probabilities of experiencing procedure-related adverse events (TUR syndrome, blood transfusion, and clot retention) based on the meta-analysis output. Procedure duration was excluded from the analysis because no statistically significant difference was observed between M-TURP and TURis in the meta-analysis. The pooled reduction in catheterisation time estimated from the meta-analysis of 0.2 d for TURis versus M-TURP was statistically significant. However, given that a patient's catheter would generally be removed while in the hospital, this reduction is not expected to result in cost savings beyond those realised through the reduction in hospital stay, which was modelled. As such, catheterisation time was not modelled explicitly. The model considered a nondefined short time horizon covering the prostate resection procedure and immediately afterwards—the period during which the complications modelled are expected to occur. Capital costs assumed equipment life spans of 7 yr. Based on findings of the SR, no difference in functional outcomes (eg, efficacy) was assumed between the procedures with respect to prostate resection weight or radicality.
Supplementary Table 1 lists all the variables included in the analysis. Sourcing of inputs, rationale, and further details are provided in Supplement 1.
Sensitivity analyses (based on predefined scenarios) were conducted. The first scenario considered all-cause readmission following a TURP procedure, based on data from the single study identified, Fagerstrom et al [23], which indicated a threefold increase in all-cause readmission for M-TURP versus TURis. This study reported readmissions as a result of bleeding, infection, and “other causes.” Because the base-case analysis included clot retention, the scenario considered readmissions from infection and other causes to avoid double counting (it is assumed that “bleeding” includes clot retention). A separate scenario considered a situation in which TURis is performed as day-case surgery.
One-way deterministic sensitivity analysis was performed in which all model parameters were varied either according to published ranges or by ±25%. Results are presented as Tornado diagrams. Probabilistic sensitivity analysis (PSA) was also conducted, in which input parameters were varied according to specified probability distributions. Threshold analysis was conducted to explore the relationship between key model parameters and model results up to the point at which the main conclusions of the model might change, that is, in this case to determine the value at which TURis would be cost neutral (ie, no longer cost saving) versus M-TURP.
The original electronic searches identified a total of 1116 publications. Following title and abstract review, 783 were excluded and 14 relevant RCTs remained, following assessment of the full paper. No unpublished studies were identified from ClinicalTrials.gov. The updated search identified a total 63 further publications, of which two full-text RCT publications remained after review of title and abstract [24] and [25]. On review of the full text, it was found that both of these publications reported data from the same trial, and only the most recent publication, reporting the most up-to-date data, was included in the analysis [24]. Figure 1 shows the study selection flow diagram.

Quality assessment of the identified RCT (Supplementary Table 2) showed that the patient population was consistent with the target population of the analysis. Study end points were clear and appropriately measured according to study design and follow-up schedule.
Six studies were found to be suitable for inclusion in the pairwise meta-analysis of the incidence of TUR syndrome [23], [26], [27], [28], [29], and [30]. The total number of events reported in the studies is presented in Table 2. There were no TUR syndrome events in the TURis arm reported in any of the studies, consistent with the use of saline. The analysis estimated a pooled relative risk (RR) for TUR syndrome of 0.18 (95% confidence interval [CI], 0.05–0.61; p = 0.006) in favour of TURis.
Table 2
Results of the meta-analysis of randomised controlled trials of transurethral resection in saline versus monopolar transurethral resection of the prostate
| Outcome | No. of studies analysed [references] | Patients, n | Events, n | RR for TURis versus M-TURP (95% CI) |
p value | ||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| TUR syndrome | 6 [19], [22], [23], [24], [25], and [26] | 767 | 734 | 13 | 0.18 (0.05–0.61) | 0.006 | |
| Blood transfusion | 7 [19], [20], [22], [23], [24], [25], and [27] | 595 | 580 | 14 | 40 | 0.34 (0.18–0.61) | 0.0003 |
| Clot retention | 6 [20], [22], [24], [25], [27], and [28] | 594 | 594 | 11 | 26 | 0.43 (0.22–0.86) | 0.0161 |
| Urethral strictures | 7 [19], [20], [22], [24], [25], [27], and [29] | 821 | 804 | 50 | 39 | 1.27 (0.85–1.9) | 0.2474 |
| Patients, n | Weighted mean | Mean difference for TURis (95% CI) |
p value | ||||
|---|---|---|---|---|---|---|---|
| TURis | M-TURP | TURis | M-TURP | ||||
| Hospital stay, d | 4 [20], [22], [23], and [29] | 490 | 478 | 2.87 | 3.43 | −0.56 (−0.77 to −0.35) | <0.0001 |
| Catheterisation time, d | 4 [20], [22], [23], and [29] | 469 | 459 | 1.87 | 2.10 | −0.23 (−0.38 to −0.09) | 0.0019 |
| Procedure time, min | 6 [20], [22], [25], [27], [29], and [30] | 676 | 654 | 55.58 | 56.53 | −0.95 (−3.35 to 1.46) | 0.439 |
CI = confidence interval; M-TURP = monopolar transurethral resection of the prostate; RR = relative risk; TUR = transurethral resection; TURis = transurethral resection in saline.
Eight RCTs reported the incidence of blood transfusion, but only seven of these were included in the meta-analysis [23], [24], [26], [27], [28], [29], and [31] (Table 2). The study by Michielsen et al was excluded from the analysis because a higher proportion of TURis procedures was conducted by trainee surgeons compared with M-TURP (32% vs 7%, respectively), resulting in increased procedure duration in this arm as well as increased blood loss and, consequently, blood transfusions [32]. Exclusion of this study on the basis of heterogeneity was accepted by the External Assessment Centre for MTG23 [19]. The results of the meta-analysis showed a statistically significant reduction in blood transfusions with use of TURis compared with M-TURP (RR: 0.34; 95% CI, 0.18–0.61; p = 0.0003).
Six studies were identified for meta-analysis of the incidence of clot retention [24], [26], [28], [29], [31], and [32]. The pooled RR was 0.43 in favour of TURis (95% CI, 0.22–0.86; p = 0.0161), demonstrating that use of TURis is associated with fewer cases of clot retention than M-TURP (Table 2).
Meta-analysis was conducted on seven studies reporting data on urethral strictures [23], [24], [26], [28], [29], [31], and [33]. A non-significantly different pooled RR of 1.27 (95% CI, 0.85–1.9; p > 0.05) was observed for TURis versus M-TURP (Table 2). As a result, this outcome was not considered in the economic analysis.
Further meta-analyses suggested that TURis is associated with statistically significant reductions in hospital stay and catheterisation time (Table 2). For these outcomes, meta-analyses were restricted to studies reporting both the mean duration and standard deviation. From a pooled analysis of four studies reporting length of hospital stay [24], [26], [27], and [33], the mean difference for TURis versus M-TURP was −0.56 d (95% CI, −0.77 to −0.35; p < 0.0001). Analysis of four studies presenting data on catheterisation time [24], [26], [27], and [33] resulted in a statistically significant mean difference for TURis versus M-TURP of −0.23 d (95% CI, −0.38 to −0.09; p = 0.0019); however, this difference is not clinically meaningful because such a minimal reduction in catheterisation time is unlikely to make a considerable difference to patients. Six studies reported procedure duration [24], [26], [29], [31], [33], and [34] and, although not significant, the resulting pooled mean difference for TURis versus M-TURP was −0.95 min (95% CI, −3.35 to 1.46; p > 0.05).
Only statistically significant differences in clinical outcomes in the meta-analysis, which directly affect costs, were considered in the base-case analysis: TUR syndrome, blood transfusions, clot retention, and length of hospital stay. Table 3 shows the results of the base-case analysis. Costs of adopting TURis differed according to whether or not hospitals owned an Olympus monopolar system. Based on 150 TURP procedures per annum, capital costs of TURis (Supplementary Table 1) for hospitals with existing Olympus systems and for those without Olympus systems were £9.68 and £29.13 per patient per year, respectively. The total cost per patient (including capital equipment, consumables, hospital stay, and complications) of using TURis for hospitals with Olympus systems in place was £881.71 versus £1085.49 for M-TURP, a cost saving of £203.78 per patient. In hospitals without Olympus systems, the total per patient cost of using TURis was £901.16 compared with £1014.58 for M-TURP, a cost saving of £113.42 per patient.
Table 3
Potential cost savings with transurethral resection in saline versus monopolar transurethral resection of the prostate*
| Cost category, £ | Costs: existing (nonexisting) Olympus centre | |||
|---|---|---|---|---|
| M-TURP | TURis | Difference | Relative difference, % | |
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Total | 1085.49 (1014.58) | 881.71 (901.16) | −203.78 (−113.42) | −19 (−11) |
| With inclusion of readmission due to all causes in the model | ||||
| Capital equipment | – | 9.68 (29.13) | 9.68 (29.13) | – |
| Procedure consumables | 137.75 (66.84) | 161.14 (161.14) | 23.39 (94.30) | 17 (141) |
| Hospital stay | 852.60 (£852.60) | 687.58 (687.58) | −165.02 (−165.02) | −19 (−19) |
| Complications | 95.14 (95.14) | 23.32 (23.32) | −71.82 (−71.82) | −75 (−75) |
| Repeat procedures | 66.70 (66.70) | 25.38 (25.38) | −41.32 (−41.32) | −62 (−62) |
| Total | 1152.19 (1081.28) | 907.09 (926.53) | −245.10 (−154.75) | −21 (−13) |
* Based on 150 procedures per annum.
M-TURP = monopolar transurethral resection of the prostate; TURis = transurethral resection in saline.
Higher equipment costs for TURis versus M-TURP were offset by savings from reductions in hospital stay and complications. Assuming a reduction in hospital stay of 0.56 d from the meta-analysis, the base-case analysis found that TURis was associated with a £165.02 reduction in the cost of hospital stay per patient versus M-TURP. TURis was also associated with a £71.82 per patient reduction in complication costs compared with M-TURP.
A further potential benefit of the TURis system is the option of conducting surgery on a day-case basis. Some UK centres are already adopting this practice [35] and [36], and the potential for further cost savings in hospital stay costs via this practice was acknowledged by NICE in MTG23 [20]. Although in clinical practice various factors influence the implementation of day-case surgery, testing a scenario reflecting this situation, in which patients undergoing TURis incurred no inpatient day costs, cost savings were £891.36 and £801.00 per patient, for Olympus and non-Olympus centres, respectively.
Considering readmissions in a further scenario, TURis was associated with annual cost savings versus M-TURP of £245.10 and £154.75 per patient, for Olympus and non-Olympus centres, respectively (Table 3). Readmission for BPH is not widely reported, thus data relating to this outcome are limited; the scenario analysis was based on a single study [23].
Sensitivity analysis also demonstrated that TURis remained cost saving versus M-TURP when each tested model parameter was varied according to its defined range. Key drivers of cost savings were reduction in hospital stay and inpatient day costs (Fig. 2). Results of PSA demonstrated that the cost-consequence analysis was robust across both types of centre. For Olympus centres, use of TURis was associated with a mean saving of £200.77 per patient (95% CI, −£115.54 to −£298.67) and remained cost saving in all 1000 simulations. For Olympus centres, the mean saving was £112.93 per patient (95% CI, £−32.94 to £−211.22), and TURis remained cost saving in 99.8% of simulations.

Results of the one-way sensitivity analysis. (A) Existing Olympus monopolar centre; (B) non-Olympus monopolar centre.
TUR = transurethral resection; TURis = transurethral resection in saline; TURP = transurethral resection of the prostate.
Results of the threshold analysis on model parameters (Table 4) confirmed the robustness of the base-case results. Existing Olympus centres would need to perform as few as seven procedures annually for TURis to become cost saving versus M-TURP. For non-Olympus centres, the number is 31. Keeping all other model parameters constant, TURis would need to be associated with significantly increased risks of blood transfusion and clot retention compared with M-TURP to cease being cost saving overall. Based on the findings of the meta-analysis, which showed statistically significant reductions in these events for TURis versus TURP, it is not considered plausible that RRs as high as those reported in Table 4 would be observed for TURis versus M-TURP.
Table 4
Results of the threshold analysis
| Variable | Base case (CI: lower–upper) |
Cost neutral: existing Olympus centre | Cost neutral: non-Olympus centre |
|---|---|---|---|
| Cohort size | 150 (100–200) | 7 patients | 31 patients |
| Probability of TUR syndrome with TURP, % | 1.77 (1.33–2.21) | NA | NA |
| Probability of blood transfusion with TURP, % | 6.90 (5.17–8.62) | NA | NA |
| Probability of clot retention with TURP, % | 4.38 (3.28–5.47) | NA | NA |
| Relative risk of blood transfusion for TURis versus TURP, % | 0.34 (0.18–0.61) | 9.32 | 5.34 |
| Relative risk of clot retention for TURis versus TURP | 0.43 (0.21–0.85) | 6.05 | 3.56 |
| Total capital cost for TURis at non-Olympus centre, £ | 26 715 (20 036–33 394) | – | 130 744 |
| Total capital cost for TURis at existing Olympus monopolar centre, £ | 8880 (6660–11 100) | 195 781 | – |
| Life span for TURis capital equipment, yr | 7 (5–10) | 0.28 | 1.30 |
| Discount rate for costs, % | 3.5 (0–6) | NA | 79 |
| Overall consumables cost for TURP per procedure, £ | Existing monopolar centre: 138 (103–172) Non-Olympus centre: 67 (50–84) |
NA | NA |
| Use of TURis rollers as a proportion of all TURis electrode use, % | 22 (17–28) | NA | 94 |
| Mean length of inpatient stay after prostate resection procedure with TURP, d | 2.9 (2.2–3.6) | NA | NA |
| Reduction in length of stay associated with TURis, d | 0.56 (0.35–0.77) | NA | 0.18 d |
| Cost per inpatient day in general ward, £ | 294 (197–344) | NA | 91.93 |
| Incremental cost for a patient experiencing TUR syndrome, £ | 2042 (1532–2553) | NA | NA |
| Incremental cost for a patient requiring a blood transfusion, £ | 329 (247–411) | NA | NA |
CI = confidence interval; NA = not applicable; TUR = transurethral resection; TURis = transurethral resection in saline; TURP, transurethral resection of the prostate.
Significant increases in the annual cost of capital equipment for TURis would be required for TURis to cease being cost saving versus M-TURP. This could only happen as a result of short equipment life span in practice because prices are generally stable over a period such as the modelled time horizon. The shortened life spans that would be required for TURis to cease being cost saving versus M-TURP (0.28 yr for existing Olympus centres and 1.30 yr for non-Olympus centres) demonstrate the robustness of the conclusions of the analysis to changes in TURis capital costs. TURis would cease being cost saving if the reduction in hospital stay for TURis versus M-TURP was removed (only for non-Olympus centres).
This study presents current and compelling clinical and economic evidence supporting adoption of the TURis system as an alternative to M-TURP, which is the current UK surgical standard of care for the treatment of BPH. The analysis was based on an SR of RCT evidence and synthesis of those data for both systems, with a subsequent economic analysis conducted from the perspective of the NHS in England and Wales. The data synthesis (by meta-analysis) showed that TURis was associated with significant improvements in perioperative safety and in duration of hospital stay compared with M-TURP. The results of the base-case economic analysis found that the adoption of TURis could result in cost savings versus M-TURP of £204 and £113 per patient for existing and non-Olympus TURP centres, respectively. Sensitivity analysis demonstrated that reduction in hospital stay was a key driver of results. Inclusion of readmissions resulted in cost savings with TURis versus M-TURP of £245 and £155 per patient for Olympus and non-Olympus centres, respectively. Although this analysis was based on a single RCT (of good quality), NICE considered it sufficiently robust [20].
Although efficacy in prostate resection surgery for treatment of voiding symptoms presumed secondary to BPH is well established, possible perioperative complications and associated costs remain a concern. Current European guidelines state that B-TURP offers similar efficacy to M-TURP but with reduced perioperative morbidity. The guidelines recommend that the choice of B-TURP should be based on surgeon experience, availability of equipment, and patient preference [6]. Although there is currently no European consensus on the use of a specific B-TURP system, in England and Wales the recently published NICE MTG23 guidance concludes that the available evidence supports the adoption of TURis for surgical treatment of BPH and that the TURis system is clinically equivalent to M-TURP, but with the advantage of reducing the risk of perioperative complications [20]. It further states that TURis has the potential to be cost saving compared with M-TURP [20].
The SR presented here demonstrates that the TURis system is supported by strong clinical data in the form of 15 good quality RCTs. To our knowledge, this is the first study to evaluate the wealth of RCT evidence in support of TURis for conducting meta-analysis to support an economic evaluation.
The findings from the economic analysis may have important implications for decision makers. The results suggest that NHS hospitals in England and Wales adopting TURis specifically as an alternative to M-TURP can expect both cost savings and improved clinical outcomes. Based on the results of the meta-analyses, treating 56 people with TURis instead of M-TURP could prevent one case of TUR syndrome. The numbers needed to treat to avoid one blood transfusion and one case of clot retention are 22 and 40, respectively. Because Hospital Episode Statistics data indicate that approximately 14 500 TURP procedures were performed out in England in 2012–2013, approximately 260 cases of TUR syndrome might be avoided if TURis was to replace M-TURP, along with 660 blood transfusions and 360 cases of clot retention. Given the complication costs outlined in Supplementary Table 1, avoiding these complications alone might result in an annual cost savings of >£1 million in England. When reductions in hospital stay are considered, the annual cost savings exceed £3.4 million. Beyond potential cost savings, this represents efficiency improvements for hospitals, enhancing patient management and throughput.
Introduction of medical devices into clinical practice is often limited by availability of high-quality clinical evidence, an essential component of robust economic evaluation of any medical intervention. Large-scale RCTs are conventionally used as the gold standard for demonstrating clinical efficacy of drug therapies; however, medical devices face a range of challenges in RCT design that often affect quality. For example, randomisation may not be possible due to ethical constraints, and blinding (especially double blinding) may not be feasible, thus increasing the risk of bias [37] and [38]. The cohort size and timescale of follow-up and assessments may also be limited [38]. Consequently, the clinical evidence base for a medical device can often be weak. In contrast, this study benefits from high-quality RCT evidence, acknowledged in the NICE MTG23 guidance, comprising 15 studies that show the clinical evidence for TURis supports the adoption of the technology. Patients included in the studies have undergone standard M-TURP or TURis procedures and no deviations in procedure protocol were observed, providing an evidence base with low heterogeneity.
A further strength of the economic analysis is that we only included those clinical outcomes for which meta-analysis showed statistically significant differences between the two treatments. Assumptions regarding resource use for complications were based on NICE clinical guideline 97 [39] and had been validated by two clinical experts for the MTEP assessment.
The analysis is not without limitations. For meta-analysis, a random-effects model is generally considered more robust because it takes heterogeneity into account. The random-effects model is only recommended, however, if ≥10 studies are available for an end point, which was not the case here. We therefore performed a fixed-effects meta-analysis. Second, complications would vary in severity; the costs considered in the analysis are intended to reflect the typical cost of treating each event. Because the model is based on UK cost data, the results are not generalizable to other countries.
The findings of the current study demonstrate that the TURis system specifically is supported by high-quality evidence. TURis demonstrates equivalent efficacy versus M-TURP, the current standard of care and is associated with statistically significant improvements in perioperative safety and duration of hospital stay. Improvements in safety with TURis could reduce the incidence of readmission following surgery. Finally, the clinical benefits of TURis have the potential to translate into cost savings for the NHS because TURis is not associated with a steep learning curve for surgeons.
Author contributions: Zenichi Ihara had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Treharne, Crowe, Booth, Ihara.
Acquisition of data: Treharne, Crowe, Booth, Ihara.
Analysis and interpretation of data: Treharne, Crowe, Booth, Ihara.
Drafting of the manuscript: Treharne, Crowe.
Critical revision of the manuscript for important intellectual content: Ihara.
Statistical analysis: Treharne.
Obtaining funding: Ihara.
Administrative, technical, or material support: Treharne, Crowe, Booth, Ihara.
Supervision: Ihara.
Other (specify): None.
Financial disclosures: Zenichi Ihara certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was funded by Olympus Europe SE & CO, which helped design and conduct the study; collect and interpret the data; and prepare, review, and approve the manuscript. No other sources provided funding throughout the conduct of the study. Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were funded by Olympus Europe SE & CO.
Acknowledgment statement: Systematic review, network meta-analysis and health economics, medical writing, and editorial support for the preparation of this manuscript were provided by DRG Abacus.