Context
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men and have multifactorial aetiology.
Objective
To develop European Association of Urology (EAU) guidelines on the assessment of men with non-neurogenic LUTS.
Evidence acquisition
A structured literature search on the assessment of non-neurogenic male LUTS was conducted. Articles with the highest available level of evidence were selected. The Delphi technique consensus approach was used to develop the recommendations.
Evidence synthesis
As a routine part of the initial assessment of male LUTS, a medical history must be taken, a validated symptom score questionnaire with quality-of-life question(s) should be completed, a physical examination including digital rectal examination should be performed, urinalysis must be ordered, post-void residual urine (PVR) should be measured, and uroflowmetry may be performed. Micturition frequency-volume charts or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia. Prostate-specific antigen (PSA) should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of symptom progression and complications. Renal function must be assessed if renal impairment is suspected from the history and clinical examination, if the patient has hydronephrosis, or when considering surgical treatment for male LUTS. Uroflowmetry should be performed before any treatment. Imaging of the upper urinary tract in men with LUTS should be performed in patients with large PVR, haematuria, or a history of urolithiasis. Imaging of the prostate should be performed if this assists in choosing the appropriate drug and when considering surgical treatment. Urethrocystoscopy should only be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment. Pressure-flow studies should be performed only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted.
Conclusions
These guidelines provide evidence-based practical guidance for assessment of non-neurogenic male LUTS. An extended version is available online ( www.uroweb.org/guidelines ).
Patient summary
This article presents a short version of European Association of Urology guidelines for non-neurogenic male lower urinary tract symptoms (LUTS). The recommended tests should be able to distinguish between uncomplicated male LUTS and possible differential diagnoses and to evaluate baseline parameters for treatment. The guidelines also define the clinical profile of patients to provide the best evidence-based care. An algorithm was developed to guide physicians in using appropriate diagnostic tests.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).
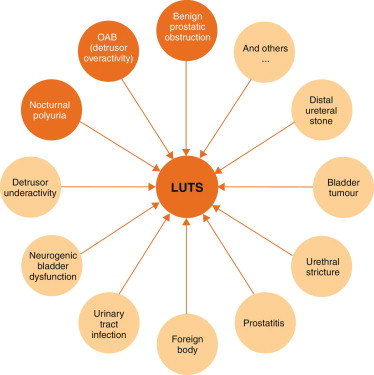
Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
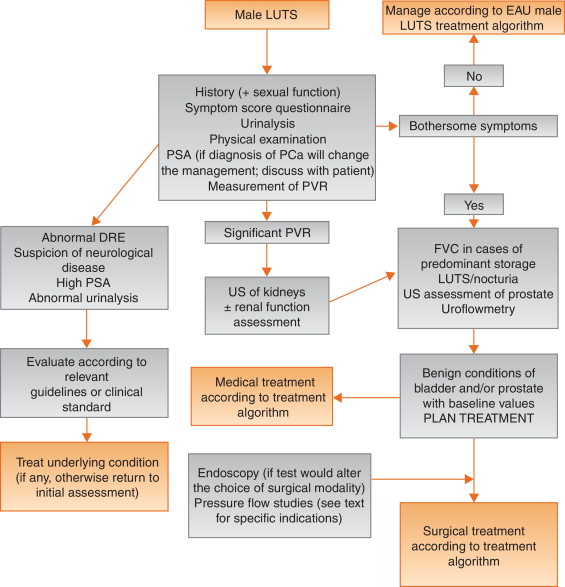
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.
Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
Lower urinary tract symptoms (LUTS) represent one of the most common clinical complaints in adult men [1] . The prevalence of LUTS increases with age, and estimates vary widely depending on definitions and cohorts studied[1] and [2]. LUTS have a major impact on health-related quality of life (QoL) [2] and are associated with substantial personal and societal costs [3] .
LUTS can be divided into storage, voiding, and post-micturition symptoms, and have traditionally been related to bladder outlet obstruction (BOO) as a result of benign prostatic obstruction (BPO), which is often caused by benign prostatic enlargement (BPE) resulting from the histologic condition benign prostatic hyperplasia (BPH) [4] . Several recent studies have shown, however, that LUTS are not necessarily related to pathologies of the prostate. For instance, various types of bladder dysfunction may also be involved in the pathogenesis of LUTS, which is sometimes urodynamically manifest as detrusor overactivity (during the storage phase) or underactivity (during the voiding phase). In addition, many other conditions, both urological and nonurological, may also contribute to LUTS ( Fig. 1 ).

Owing to the high prevalence of LUTS and the underlying multifactorial pathophysiology, accurate assessment of male LUTS is crucial to establish a differential diagnosis among possible causes and to define the clinical profile of men with LUTS to provide the best evidence-based care (overall objectives). The assessment should be able to identify patients for whom watchful waiting (WW) or medical or surgical treatment can be recommended, as well as men at risk of disease progression, and to assess patients’ values and preferences. The guidelines aim to answer the clinical question as to which tests are recommended in the assessment of non-neurogenic LUTS in men aged ≥40 yr and when these tests should be performed.
The recommendations in these guidelines are based on a structured literature search for articles published in English according to the PubMed/Medline, Web of Science, and Cochrane databases between 1966 and October 1, 2013, including the search terms “lower urinary tract symptoms”, “benign prostatic hyperplasia”, “detrusor overactivity”, “overactive bladder”, “nocturia”, and “nocturnal polyuria” in combination with the prespecified diagnostic tests and the search limits “humans”, “adult men”, “review”, “randomised clinical trials”, “clinical trials”, and “meta-analysis”. Each extracted article was separately analysed, classified, and labelled with a level of evidence (LE) according to a classification system modified from the Oxford Centre for Evidence-based Medicine, ranging from systematic reviews of randomised trials (LE 1a, highest evidence level) to expert opinion (LE 4, lowest evidence level) (modified from [5] ).
The working panel used the Delphi technique consensus approach, which is based on the rationale that decisions captured systematically from a structured group of individuals (the working panel) are more valid than those from unstructured groups. When published information is scarce, experts can make inferences using other data from comparable contexts. Using bespoke software ( www.acord.it ), propositions were put to experts, who voted for their preference. The results from the group were then sent back anonymously to all participants, who were able to review their responses in the context of group-wide results. This practice conferred anonymity and allowed opinions to be expressed free from peer-group pressure. The web-based system offered participants the option to comment and justify their decisions anonymously. After consideration of the view of the group and a review of the comments, a second round of anonymous voting took place. Experts were encouraged to revise their earlier answers in light of the replies of other working panel members. Three iterations of the process were used, during which the range of the answers decreased and the group converged towards a consensus answer. The working panel predetermined the consensus level at 77% (7 out of 9) using the Delphi process, such that consensus on and recommendation for any test required support from at least 77% of the panel members. The panel has classified diagnostic tests into three categories: must, should, and may, which represents the highest, intermediate, and lowest levels of obligation, respectively.
Each recommendation is based on the strongest clinically relevant data as far as possible. However, it should be noted that when recommendations are graded, there is no automatic relationship between LE and grade of recommendation (GR). The availability of randomised controlled trials (RCTs) may not necessarily translate into a grade A recommendation if there are methodological limitations, disparity in published results, uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in patients’ values and preferences, or uncertainty about whether the intervention represents wise use of resources. Alternatively, an absence of high-level evidence does not necessarily preclude a grade A recommendation; if there is considerable clinical experience and consensus to support a high-level recommendation, a grade A recommendation can be made. Such decisions are clearly indicated in Table 1 with an asterisk to denote “upgraded based on panel consensus”.
Table 1 Level of evidence and grade of recommendation for the assessment of non-neurogenic male lower urinary tract symptoms
| Assessment tool | LE | GR |
|---|---|---|
| A medical history must always be taken from men with LUTS | 4 | A * |
| A validated symptom score questionnaire with QoL question(s) should be used for routine assessment of male LUTS in all patients and should be applied for re-evaluation of LUTS during treatment | 3 | B |
| Micturition FVCs or bladder diaries should be used to assess male LUTS with a prominent storage component or nocturia | 3 | B |
| FVCs should have a duration of at least 3 d | 2b | B |
| Physical examination including DRE should be a routine part of the assessment of male LUTS | 3 | B |
| Urinalysis (by dipstick or urinary sediment) must be used in the assessment of male LUTS | 3 | A * |
| PSA should be measured only if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of progression of BPE | 1b | A |
| Renal function must be assessed if renal impairment is suspected based on history and clinical examination, if hydronephrosis is present, or when considering surgical treatment for male LUTS | 3 | A * |
| Measurement of PVR in male LUTS should be a routine part of the assessment | 3 | B |
| Uroflowmetry in the initial assessment of male LUTS may be performed and should be performed before any treatment | 2b | B |
| Imaging of the upper urinary tract (with US) in men with LUTS should be performed in patients with a large PVR, haematuria, or a history of urolithiasis | 3 | B |
| When considering medical treatment for male LUTS, imaging of the prostate (either by TRUS or transabdominal US) should be performed if it assists choice of the appropriate drug | 3 | B |
| When considering surgical treatment, imaging of the prostate (either by TRUS or abdominal US) should be performed | 3 | B |
| Urethrocystoscopy should be performed in men with LUTS to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change treatment | 3 | B |
| PFS should be performed only in individual patients for specific indications before surgery or when evaluation of the underlying pathophysiology of LUTS is warranted | 3 | B |
| PFS should be performed in men who have had previous unsuccessful (invasive) treatment for LUTS | 3 | B |
| When considering surgery, PFS may be used for patients who cannot void >150 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men with PVR >300 ml | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS may be performed in men aged >80 yr | 3 | C |
| When considering surgery in men with bothersome predominantly voiding LUTS, PFS should be performed in men aged <50 yr | 3 | B |
* Upgraded based on panel consensus.
BPE = benign prostatic enlargement; FVC = frequency/volume chart; GR = grade of recommendation; LE = level of evidence; LUTS = lower urinary tract symptoms; PFS = pressure-flow study; PSA = prostate-specific antigen; PVR = post-void residual urine; QoL = quality of life; TRUS = transrectal US; US = ultrasound.
The working panel for the non-neurogenic male LUTS guidelines consists of experts with a urological and epidemiological background. Although the guidelines are written primarily for urologists, they can also be used by general practitioners, patients, and other stakeholders. The working panel intends to regularly update the content and recommendations according to the structure and classification systems given.
Recommendations apply to men aged ≥40 yr who seek professional help for various non-neurogenic benign forms of LUTS. Men with LUTS not falling into this category (eg, concomitant neurological diseases, young age, prior lower urinary tract disease or surgery) usually require a more extensive work-up that is not covered by these guidelines but may include several of the tests mentioned in the following section. All recommendations for diagnostic tests, along with LE and GR, are summarised in Table 1 .
Earlier guidelines on male LUTS and/or BPH emphasise the importance of assessing the patient's history[6], [7], [8], and [9]. The aim of obtaining a medical history is to identify potential causes of LUTS and relevant comorbidities, such as medical (eg, diabetes mellitus or insipidus, renal disease, heart failure, sleep apnoea) and neurological diseases (eg, Parkinson's disease, multiple sclerosis, cerebrovascular disease, spinal cord injury, or prolapsed intervertebral disc impinging on the spinal cord). It is further recommended to review current medication, and assess lifestyle habits, as well as emotional and psychological factors. The panel highlights the need to discuss the patient's perspectives regarding LUTS and possible treatment options. The patient should be reassured that the presence of LUTS does not indicate a higher prevalence of prostate cancer (PCa) compared with asymptomatic men[10] and [11].
As part of the urological/surgical history, a self-completed validated symptom questionnaire (Section 3.2 ) should be delivered to objectively identify and quantify LUTS. The same symptom questionnaire should subsequently be discussed with the patient during follow-up to assess therapeutic efficacy. Potential erectile and other forms of sexual dysfunction should be investigated (preferably with validated symptom questionnaires).
During the past two decades, symptom scores have become a standard tool in the assessment of male LUTS. Existing guidelines on male LUTS and/or BPH recommend the use of validated symptom score questionnaires[6], [7], [8], and [9]. Several questionnaires are available, all of which are sensitive to symptom changes and treatment monitoring[12], [13], [14], [15], [16], [17], and [18].
The International Prostate Symptom Score (IPSS) is an eight-item (seven symptom questions and one global QoL question) questionnaire, initially created as the American Urological Association Symptom Index [14] . The International Consultation on Incontinence Questionnaire ICIQ-MLUTS was created from the ICS male questionnaire (which resulted from an outcome of the ICS BPH study) and is another widely used and validated patient-completed questionnaire for evaluating male LUTS [15] . A third questionnaire is the Danish Prostate Symptom Score (DAN-PSS) [13] , which is mainly used in Denmark and Finland. The IPSS includes only one overall QoL question, whereas the DAN-PSS and ICIQ-MLUTS assess the bother of individual LUTS.
Symptom scores are recommended for all patients during initial assessment as they are helpful in quantifying individual LUTS and identifying which type of symptoms (storage or voiding) are predominant, yet they are not disease-, age-, or gender-specific. Symptom scores can also be used to monitor response to therapy.
Recording of the volume and time of each void by the patient is referred to as a frequency-volume chart (FVC). The record is known as a bladder diary if additional information is captured, such as fluid intake, use of pads, activities during recording, or symptom scores [4] . Parameters that can be derived from the FVC include: voiding frequency per 24 h; total voided volume per 24 h, including the fraction of urine produced during the night, known as the nocturnal polyuria index; and the volume of individual voids (mean and range).
FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s)[19], [20], and [21]. FVCs are typically more accurate than patient recall[22] and [23], particularly for nocturia. However, FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed [24] . Hence, there is no agreement on standardising the approach to deriving the above information in LUTS evaluation [25] .
The observation duration should be long enough to avoid sampling errors, but short enough to avoid noncompliance [25] . Several studies have compared shorter (3–5 d) with longer (7 d) diary durations[26], [27], [28], [29], [30], and [31]. A 2009 systematic review of the literature recommended the use of ≥3 d [32] . A recent phase 1 study on the development and validation of a urinary diary suggested that the FVC duration should be ≥4 d [33] .
A physical examination should be performed on the suprapubic area to rule out bladder distention, on the external genitalia to identify conditions that may cause or contribute to LUTS (eg, urethral discharge, phimosis, meatal stenosis, penile cancer), and on the perineum/lower limbs to evaluate motor/sensory function. Therefore, a physical examination is especially useful for differential diagnosis of LUTS.
DRE is an important examination in men with LUTS and may help to determine the coexistence of PCa, despite its low diagnostic value, and abnormalities of anal sphincter tone. DRE overestimates prostate volume (PV) in smaller prostates and underestimates PV in larger prostates, but is a sufficient method to discriminate whether PV is greater or less than 50 ml [34] . The capacity of DRE to estimate PV is helpful for choosing treatment options, as these depend on PV (eg, 5α-reductase inhibitors [5-ARIs], transurethral incision of the prostate, transurethral resection of the prostate, and others; see EAU Guidelines on the treatment of non-neurogenic male LUTS [35] ).
Urinalysis (dipstick or sediment) is an inexpensive test that does not require sophisticated technical equipment, and it must be incorporated in the primary evaluation of any patient presenting with LUTS to determine conditions such as urinary tract infection and diabetes mellitus on the basis of abnormal findings (haematuria, proteinuria, pyuria, glucosuria, ketonuria, positive nitrite test). Therefore, urinalysis is helpful for the differential diagnosis of LUTS. Once abnormal findings have been diagnosed, further evaluation is recommended according to the standards provided in other EAU guidelines, such as those on non–muscle-invasive bladder cancer, muscle-invasive and metastatic bladder cancer, upper urinary tract urothelial cell carcinoma, primary urethral carcinoma, and urological infections[36], [37], [38], and [39].
Urinalysis is traditionally recommended in most guidelines for the primary management of patients with LUTS[40] and [41]. Even in the absence of controlled studies, there is general expert consensus that the benefits clearly outweigh the costs, although the use of urinalysis should always be associated with prognostic significance [42] . Nevertheless, despite official guidelines and the widespread use of urinalysis among urologists [43] , the value of urinary dipstick/microscopy for diagnosing urinary tract infection in patients with painless LUTS has recently been questioned [44] .
Several reports have demonstrated the reliability of serum PSA for predicting PV[45], [46], and [47]. However, determination of exact PV for an individual from PSA does not seem to be possible because of the relatively large standard deviation for the estimation curve [48] .
The role of serum PSA in the diagnosis of PCa is described in the EAU guidelines on prostate cancer [49] . The benefits and harms of using serum PSA testing to diagnose PCa in men with LUTS should be discussed with the patient, including the possibilities of false-positive and false-negative results, complications of subsequent transrectal ultrasound (TRUS)-guided biopsy, false-negative biopsies, and overdiagnosis and overtreatment of PCa [49] .
Serum PSA appears to be a stronger predictor of prostate growth than PV [50] . In addition, the PLESS study showed that PSA also predicted changes in LUTS, QoL/bother, and the maximum urinary flow rate (Qmax) [51] . In a longitudinal study of men managed conservatively, serum PSA was a highly significant predictor of clinical progression [52] . More importantly, in the placebo arms of large double-blind controlled studies, baseline serum PSA consistently predicted the risk of acute urinary retention (AUR) and BPE-related surgery[53] and [54]. Patients with BPO appear to have higher serum PSA and greater PV compared to men without BPO [55] . The positive predictive value (PPV) of PSA for detection of BPO was recently shown to be 68% [56] .
Renal function may be assessed by measurement of serum creatinine or calculation or determination of the estimated glomerular filtration rate (eGFR). Hydronephrosis, renal insufficiency, and urinary retention appear with greater prevalence in patients with symptoms or signs of BPO [57] . Even though BPO may be partly responsible for these complications, there is no conclusive evidence that BPO is the primary cause [57] . One study evaluated 246 men presenting with LUTS and found that 11% had renal insufficiency [58] . The same study also noted that it was rather rare to find patients with high creatinine levels due to BPO alone [58] . Comiter et al [59] reported that voiding dysfunction of a non-neurogenic aetiology did not appear to be a risk factor for elevated creatinine levels. In addition, in the MTOPS study, fewer than 1% of men with LUTS presented with renal insufficiency during the observational period of at least 4 yr [54] . In 2741 consecutive patients who presented with LUTS, a decrease inQmaxand a history of hypertension and/or diabetes were significantly associated with chronic kidney disease [60] . A recent study demonstrated thatQmaxcorrelated significantly with GFR in middle-aged men with moderate to severe LUTS[61] and [62]. In addition, patients with renal insufficiency have a higher risk of developing postoperative complications compared to those with normal renal function [63] .
Post-void residual urine (PVR) can be measured by transabdominal ultrasonography, a bladder scan, or catheterisation. The interval between voiding and PVR measurement should be short [64] . Ultrasound (US) bladder volume measurement is generally the preferred approach for measuring PVR [64] , which is not necessarily associated with BOO, since high PVR can be a consequence of BOO and/or poor detrusor function (underactivity)[65] and [66].
It has been shown that for volumes >50 ml, the diagnostic accuracy of PVR measurement has PPV of 63% and a negative predictive value (NPV) of 52% in determining BOO [62] . A large PVR is not a contraindication for watchful waiting or medical therapy, although PVR indicates bladder dysfunction and predict a poor response to treatment, especially to WW. In both the MTOPS and ALTESS studies, high baseline PVR was associated with an increased risk of symptom deterioration[53] and [54]. In addition, monitoring of PVR changes over time could predict AUR occurrence; patients who subsequently developed AUR showed a steady increase in PVR [53] . This is of particular importance for the treatment of patients using antimuscarinic medication. By contrast, baseline PVR has little prognostic value for the risk of BPE-related invasive therapy in patients on α1-blocker therapy or WW [67] . However, owing to large test-retest variability and a lack of outcome studies, it is currently impossible to establish a PVR threshold for treatment decisions.
Urinary flow rate assessment is a basic noninvasive urodynamic test that is widely used to evaluate the joint functioning of the lower urinary tract components (bladder and outlet). Key parameters areQmax, voided volume, and flow pattern. Uroflowmetry parameters should ideally be evaluated when the voided volume is >150 ml.Qmaxcan be subject to within-subject variation on the same or different days[68] and [69]; therefore, it is advisable to repeat uroflowmetry measurements when the voided volume is <150 ml orQmaxor the flow pattern is abnormal.
The diagnostic accuracy of uroflowmetry for detecting BOO varies considerably and is substantially influenced by diagnostic threshold values. AQmaxthreshold of 10 ml/s had specificity of 70%, PPV of 70%, and sensitivity of 47% for BOO. For aQmaxthreshold of 15 ml/s, specificity was 38%, PPV was 67%, and sensitivity was 82% [70] ; thus, uroflowmetry alone is unsuitable for detection and quantification of BOO. LowQmaxcan arise as a consequence of BOO [71] , detrusor underactivity, or an underfilled bladder [72] . Thus, uroflowmetry is limited as a diagnostic test as a consequence of the inability to discriminate underlying mechanisms in men with lowQmax.
Specificity can be improved by repeated flow-rate testing in individual patients. Uroflowmetry can be used to monitor treatment outcomes [73] and correlate symptoms with objective findings.
Routine imaging of the upper urinary tract in men with LUTS is not recommended as these patients are not generally at higher risk of upper tract malignancy or other abnormalities (including hydronephrosis, measurable degrees of renal insufficiency, renal cysts) compared to the general population (see above)[74], [75], [76], and [77].
Several arguments support the use of renal US in preference to intravenous urography (IVU). US allows better characterisation of renal masses, the possibility of investigating the liver and retroperitoneum at the same time, and evaluation of the bladder, PVR and prostate compared to IVU, at lower cost and without radiation exposure and side effects [75] .
Imaging of the prostate can be performed using several imaging techniques including transabdominal US, TRUS, computed tomography (CT), and magnetic resonance (MR) imaging. In daily practice, however, imaging of the prostate by TRUS or transabdominal US is mainly used [75] .
PV measurement is important before treatment with 5-ARIs and for selection of an appropriate interventional treatment [35] . Recent data suggest that PV may predict which patients with LUTS will develop symptom progression and complications [54] . A large body of evidence documents the accuracy of TRUS in calculating PV. TRUS is superior to suprapubic (transabdominal) PV measurement because all three distances for the prostate can be measured more accurately via the transrectal approach[78] and [79]. The presence of a middle lobe protruding into the bladder may guide the treatment choice in patients scheduled for a minimally invasive approach.
US measurement of intravesical prostatic protrusion (IPP) has also been introduced. The concept is that a prostate median lobe protruding into the bladder can cause a valve ball type of BPO with incomplete opening and disruption of the funnelling effect of the bladder neck [80] . IPP correlated well with BPO, with PPV of 94% and NPV of 79% [80] , and also seems to predict successful outcome of trial without catheter after AUR[81] and [82]. Therefore, IPP may be a feasible option for diagnosing BPO in men with LUTS, but its role as a noninvasive alternative to pressure-flow studies (PFS) in the assessment of male LUTS is under evaluation, and currently no specific recommendations can be made.
For bladder wall thickness (BWT) assessment, the entire diameter of the bladder wall is measured, which represents the distance between the hyperechogenic mucosa and the hyperechogenic adventitia. For detrusor wall thickness (DWT) assessment, only the hypoechogenic detrusor sandwiched between the hyperechogenic mucosa and adventitia is measured [83] . It has been shown that BWT and DWT measurements have higher diagnostic accuracy in detecting BOO thanQmaxin free uroflowmetry or measurements of PVR, PV, or symptom severity [62] .
Disadvantages of the method include the lack of standardisation in terms of threshold values and bladder filling so far, with varying results for different bladder filling levels, and a lack of evidence of whether BWT or DWT is more clinically relevant [84] . The concept of bladder weight (BW) as a measure of bladder wall hypertrophy has also been introduced [85] . Comparison of UEBW and PFS revealed that UEBW could identify BOO with a diagnostic accuracy of 86.2% using a cutoff value of 35 g [86] . Measurement of BWT or DWT and UEBW may be a feasible option for diagnosing BOO in men with LUTS. The role of BWT, DWT, and UEBW as a noninvasive alternative to PFS in the assessment of male LUTS or BOO is under evaluation, and currently no specific recommendations can be made.
Patients with a history of microscopic or gross haematuria, urethral stricture (or relevant risk factors, such as history of urethritis, urethral injury, urethral instrumentation, or previous urethral surgery), or bladder cancer who present with LUTS should undergo urethrocystoscopy during diagnostic evaluation.
Several studies have addressed whether urethrocystoscopy findings correlate with functional data[87], [88], and [89]. In the largest study, urethroscopic findings were correlated to urodynamic studies in 492 elderly men with LUTS [89] . Correlation between cystoscopic appearance (grade of bladder trabeculation and grade of urethral occlusion) and urodynamic indices, detrusor overactivity, and low compliance was observed. It should be noted, however, that BOO was present in approximately 15% of patients with normal cystoscopic findings, while approximately 8% of patients had no BOO even in the presence of severe trabeculation [89] .
Evaluation of a prostatic middle lobe in urethrocystocopic findings is necessary to determine the indication for certain interventional treatments, such as transurethral needle ablation and transurethral microwave therapy.
In male LUTS, the most widespread urodynamic techniques used are filling cystometry (to assess the bladder storage phase) and PFS (to assess the voiding phase). The major aims of urodynamics are to explore the functional mechanisms of LUTS and identify potential risk factors for adverse outcomes (for informed/shared decision-making). Most parameters and diseases or conditions (eg, detrusor overactivity, low compliance, BOO/BPO, detrusor underactivity) are identified by urodynamic investigation.
PFS are the basis for identifying BOO and are the primary objective in ascertaining its presence. BOO involves increased detrusor pressure and decreased urinary flow during voiding. BOO/BPO has to be differentiated from detrusor underactivity, which is defined as decreased detrusor pressure during voiding in combination with a decreased urinary flow rate. During the storage phase, urodynamic testing of overactive bladder (OAB) patients may identify detrusor overactivity (DO), which is a urodynamic observation characterised by involuntary detrusor contractions during the filling phase, which may be spontaneous or provoked. OAB is diagnosed from the patient's symptoms, based on the presence of urgency, usually with increased daytime frequency, nocturia, and/or urgency incontinence [4] . Thus, the terms OAB and DO are not interchangeable. For instance, in one study, 21% of men with urinary urgency did not have DO [90] , and DO can be asymptomatic; several studies have described an association between BOO and DO[91] and [92].
In men with LUTS attributed to BPE, DO was present in 61% of patients (n = 1418) and independently associated with BOO grade and ageing. As BOO grade and patient age increased, DO prevalence increased, ranging from 50% in men without BOO to 83% in men with the most severe BOO [93] . Prevalence estimates of detrusor underactivity in men with LUTS vary between 11% and 40%[93] and [94]. Detrusor contractility does not appear to decline in long-term BOO, and surgical relief of BOO does not improve contractility[95] and [96]. No randomised studies were identified regarding the usefulness of cystometry in guiding clinical management for patients with LUTS. Furthermore, there are no published RCTs comparing standard investigation (uroflowmetry and PVR measurement) with PFS in men with LUTS and possible BPO.
Owing to the invasive nature of urodynamic testing because of catheter placement, computer urodynamic investigation is generally only offered once conservative treatment has failed. The panel attempted to suggest specific indications for PFS based on age, findings from other diagnostic tests, and previous treatments. These include situations in which the diagnosis of BPO is uncertain and the patient has a significant chance of additional problems such as detrusor overactivity or underactivity. The panel allocated different degrees of obligation for PFS in men >80 yr and men <50 yr, and this may reflect the lack of clear evidence ( Table 1 ). In addition, there was no consensus on whether PFS should or may be performed when considering surgery in men with bothersome predominantly voiding LUTS andQmax>10 ml/s, although the panel recognised that BOO is likely forQmax<10 ml/s and PFS are not necessarily needed. It should be underlined that patients with neurological disease, including those with previous radical pelvic surgery, should be assessed according to the EAU guidelines on neurogenic lower urinary tract dysfunction [97] .
Inclusion of intermittent synchronous x-ray imaging and filling of the bladder with contrast medium for cystometry and PFS is termed videourodynamics. The test provides additional anatomical information. During filling, imaging is usually undertaken in the postero-anterior axis and can show bladder configuration (bladder trabeculation and diverticula), vesico-ureteral reflux, or pelvic floor activity. During voiding, a 45° lateral projection is typically used and can show the exact location of BOO. Videourodynamics may be used when there is uncertainty regarding the mechanisms of voiding LUTS.
Tests are useful for diagnosis, monitoring, assessment of the prognosis for disease progression, treatment planning, prediction of treatment outcome, and ascertainment of patient values and preferences. Standardisation of LUTS assessment in men represents a significant challenge because of the low LE of existing studies. The guidelines presented here are not an update of the BPH guidelines published in 2004. The multifactorial view of the aetiology of LUTS has been adopted and a broader approach to the assessment of men suffering from LUTS has been introduced. In addition, for the first time in the male LUTS guidelines, the panel used the Delphi consensus method to strengthen the value of its recommendations. A practical algorithm based on the recommendations has been developed ( Fig. 2 ). It should also be noted that the low LE for the majority of diagnostic tests emphasises the need for high-LE studies to determine the value of each diagnostic tool.

Author contributions:Stavros Gravas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Acquisition of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Analysis and interpretation of data:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Drafting of the manuscript:Gratzke, Gravas.
Critical revision of the manuscript for important intellectual content:Gravas, Gratzke, Bachmann, Descazeaud, Drake, Madersbacher, Mamoulakis, Oelke, Tikkinen.
Statistical analysis:Gravas.
Obtaining funding:None.
Administrative, technical, or material support:Gravas, Gratzke.
Supervision:Gravas.
Other:None.
Financial disclosures:Stavros Gravas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Stavros Gravas has received grants or research support from Pierre Fabre Medicament and GSK, travel grants from Angelini Pharma Hellas, Astellas, GSK, and Pierre Fabre Medicament, and speaker honoraria from Angelini Pharma Hellas, Pierre Fabre Medicament, Lilly, and GSK, and is a consultant for Pierre Fabre Medicament and GSK. Christian Gratzke has received grants or research support from MSD, Bayer Healthcare, AMS, and Recordati, fellowship and travel grants from DFG and EUSP, and speaker honoraria from Rottapharm-Madaus, Astellas Pharma, GSK, AMS, and Steba, and is a consultant for Rottapharm-Madaus, Astellas Pharma, Lilly, Recordati, Bayer, and Dendreon. Alexander Bachmann has received grants or research support from AstraZeneca and Pfizer, and speaker honoraria from AMS, Ferring, and Bayer, has participated in trials by AstraZeneca, Pfizer, and AMS, and is a consultant for AMS, Orionpharma, Schering, Olympus, and Caris Life. Aurelien Descazeaud has received speaker honoraria from Takeda, GSK, Pierre Fabre, and Lilly, has participated in trials by Olympus, Lumenis, AMS, Allergan, Recordati, Takeda, and Pierre Fabre, and is a consultant for Sanofi, Pierre Fabre, Lilly, and Recordati. Marcus J. Drake has received grants or research support from Ferring and Astellas, fellowship or travel grants from Astellas, honoraria or consultation fees from Allergan, Astellas, and Apogepha, and speaker honoraria from Apogepha, Ferring, Pfizer, Astellas, and Allergan, has participated in trials by Allergan and Astellas, and is a consultant for J&J. Stephan Madersbacher has received speaker honoraria from Lilly, Takeda, Astellas, MSD, GSK, and Böhringer Ingelheim, and is a member of the advisory boards for Lilly, Astellas, Takeda, and GSK. Charalampos Mamoulakis has received grants or research support from Porge-Coloplast and Ariti, honoraria or consultation fees from Astellas, fellowship or travel grants from Karl Storz Endoscope, Porge-Coloplast, Cook Medical, Boston Scientific, and Astellas, and a speaker honorarium from GSK, and has participated in trials by Medivation, Karl Storz Endoscope, Eli Lilly, and Astellas. Matthias Oelke has received grants or research support from Pfizer, Astellas, and Ferring, fellowship or travel grants from Astellas, Apogepha, Recordati, Eli-Lilly, GSK, Pfizer, and Mundipharma, honoraria or consultation fees from Astellas, Allergan, and Teva, and speaker honoraria from Allergan, Pfizer, Bayer Healthcare, Eli-Lilly, GSK, Ferring, and Astellas, has participated in trials by Ferring, Apogepha, Pfizer, Astellas, Allergan, Eli-Lilly, and GT-Urological, is a consultant for Apogepha, Teva, Sophiris, GT-Urological, Recordati, Pfizer, Mundipharma, Eli-Lilly, Biocompatibles, GSK, and Astellas. Kari A.O. Tikkinen has nothing to disclose.
Funding/Support and role of the sponsor:None.
This article presents a short version of the European Association of Urology guidelines for non-neurogenic male lower urinary tract symptoms (LUTS). The analysis was performed by an eminent group of urologists with a large experience in the field of male LUTS. Some of the authors have specific expertise in the diagnosis and treatment of patient with significant nocturia.
Evidence acquisition was performed based on a structured literature search and only articles with the highest available level of evidence were selected. The Delphi technique consensus approach was used to develop the recommendations.
The authors indicate that, as a routine part of the initial assessment of male LUTS, a medical history must be taken (this includes the suspected number of nocturnal voids), a validated symptom score questionnaire with quality-of-life question(s) should be completed (however, they mention general questionnaires and do not mention specific questionnaires aiming at nocturia), a physical examination including digital rectal examination should be performed, urinalysis must be ordered, post-void residual urine (PVR) should be measured, and uroflowmetry may be performed.
As a general statement the authors indicate that micturition frequency-volume charts (FVC) or bladder diaries should be used to assess the male patients that present with LUTS with a prominent storage component or nocturia. They specifically mention that FVCs are beneficial when assessing patients with bothersome storage LUTS, particularly nocturia, as they can underpin categorisation of the underlying mechanism(s). It is also mentioned that FVCs are more accurate than patient recall, particularly for nocturia. However, they warn that FVC use may lead to a bladder training effect, and nights during FVC recording may be atypical since substantial variations in the frequency of nocturnal voids have been observed. Hence they conclude that there is no agreement on standardising the approach to deriving the above information in LUTS evaluation.
Of course, they mention that prostate-specific antigen (PSA) should be measured if a diagnosis of prostate cancer will change the management or if PSA can assist in decision-making for patients at risk of symptom progression and complications.
These guidelines advise that renal function be assessed if renal impairment is suspected based on the history and clinical examination, if the patient has hydronephrosis, or when considering surgical treatment for male LUTS.
Further recommendations are:
Based on these guidelines, urethrocystoscopy should only be performed in men with LUTS in order to exclude suspected bladder or urethral pathology and/or before minimally invasive/surgical therapies if the findings may change the treatment.
Concerning pressure-flow studies, the authors state that they should be performed only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted.
Based on the gathered information, the authors conclude that the recommended tests should be able to distinguish between uncomplicated male LUTS and possible differential diagnoses and to evaluate baseline parameters for treatment. This applies definitely for the male patient with clinically significant nocturia.
We can conclude that these guidelines provide evidence-based practical guidance for the assessment of non-neurogenic male LUTS. An extended version is available online (www.uroweb.org/guidelines).