Background
Little is known about drug adherence in men treated for lower urinary tract symptoms (LUTS). Benign prostatic hyperplasia (BPH) is one of the causes of LUTS.
Objective
To examine adherence to pharmacological therapy and its clinical value in men with LUTS.
Design, setting, and participants
Population-based cohort study using an administrative prescription database and hospital discharge codes for 1.5 million men aged ≥40 yr treated with alpha blockers (ABs) and 5-alpha reductase inhibitors (5ARIs) alone or in combination (CT).
Interventions
Therapy with ABs and/or 5ARIs.
Outcome measurements and statistical analysis
The 1-yr and long-term adherence; hospitalization rates for BPH and BPH surgery. Multivariate Cox proportional hazards regression model, propensity score matching, and sensitivity analyses.
Results and limitations
The 1-yr adherence was 29% in patients exposed to at least 6-mo therapy. Patients on CT had a higher discontinuation rate in the first 2 yr compared to those on monotherapy (p < 0.0001). Overall hospitalization rates for BPH and BPH surgery were 9.04 and 12.6 per 1000 patient-years, respectively. A lower risk of hospitalization was observed for 5ARI compared to AB therapy (hazard ratio [HR] 0.46 and 0.23; p < 0.0001). CT was associated with a reduced risk of hospitalization for BPH surgery (HR 0.94; p < 0.0001) compared to AB. Discontinuation of drug treatment was an independent risk factor for hospitalization for BPH and BPH surgery (HR 1.65 and 2.80; p < 0.0001) regardless of therapeutic group. Limitations include the paucity of clinical measures and the absence of patient-reported outcomes.
Conclusions
Adherence to pharmacological therapy for BPH is low and could affect clinical outcomes. Long-term 5ARI and CT use was associated with an independent reduced risk of hospitalization for BPH surgery. Our findings suggest the need for new strategies to increase patient adherence to prescribed treatment and more appropriate prescribing by physicians.
Patient summary
Our research shows that adherence to prescribed pharmacological therapy is crucial in the management of patients suffering from lower urinary tract symptoms. Moreover, pharmacological therapy can prevent disease progression.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.
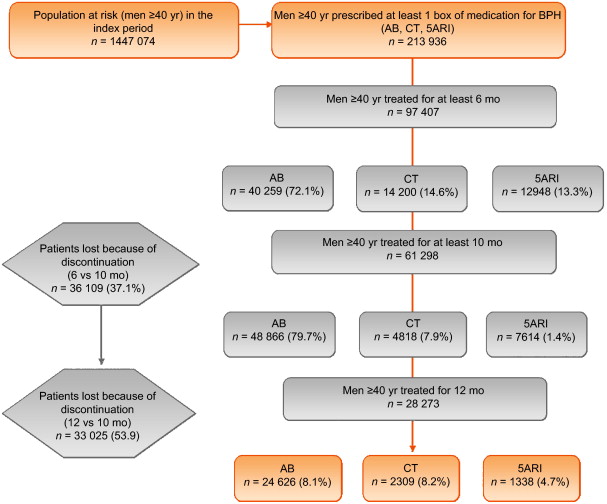
General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.
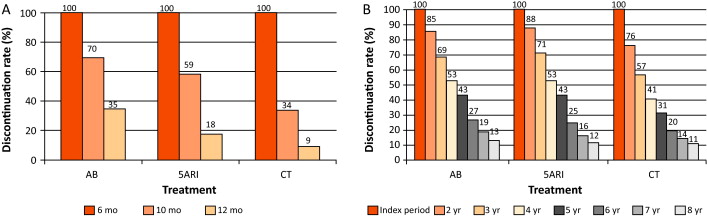
Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
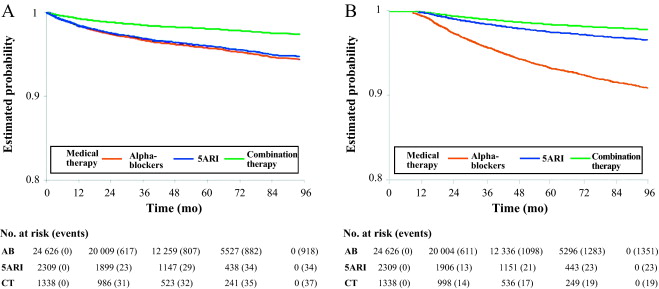
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.
Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.
Benign prostatic enlargement (BPE) is common in older men and is caused by a histopathologic condition: benign prostatic hyperplasia (BPH). Clinical manifestations of BPH include symptoms, signs, and sequaele of bladder outlet obstruction caused by BPE [1] . The prevalence of moderate-to-severe lower urinary tract symptoms (LUTS) in the community is high, ranging from 22% among 50–59-yr-old men to 45% among those in their seventh decade. Only 19% of men suffering from LUTS due to BPE seek medical treatment and only 10.2% are pharmacologically treated [2], [3], and [4].
The aim of pharmacological therapy for BPH is to improve the patient's quality of life by relieving urinary symptoms and preventing the development of complications. International guidelines suggest that patients with moderate-to-severe LUTS are best managed initially with drugs [5] and [6].
Five classes of drugs are available: phytotherapeutics, alpha blockers (ABs), 5-alpha reductase inhibitors (5ARIs), phosphodiesterase inhibitors, and antimuscarinics/beta3 agonists. Long-term combination therapy (CT) with ABs and 5ARIs is beneficial in terms of symptoms control and disease progression [7], [8], and [9].
Although pharmacological treatment of BPH is a success story in urology, daily practice suggests that several medical needs remain unmet. Whether or not this is because of limited drug effects, inappropriate patient management, or low drug adherence remains unclear [4], [10], and [11]. Drug regimens for LUTS related to BPH (LUTS/BPH) are particularly complex for several reasons (geographic, societal and cultural differences, medication costs, and local health policies).
There is little evidence available on the impact of long-term treatment and drug adherence on BPH progression in real life [12] . Data on adherence are of importance to understand possible unmet needs, to explore patient preferences, and to identify areas of intervention in health systems [13] and [14].
The aim of this study was to evaluate drug adherence and long-term clinical outcomes in patients under pharmacological therapy for LUTS/BPH.
A population-based cohort study was conducted using record-linkage analysis of three databases: a drug prescriptions database, a civil registry, and hospital discharge records (HDRs) for 6.5 million subjects across 22 local Italian health authorities.
All Italian citizens have equal access to health care services; hospital and pharmaceutical services are provided free or at a minimum charge as part of the National Health Service (NHS). The Italian national drug database includes information on prescriptions reimbursed by the NHS; drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification [15] and qualified with respect to dosage and date of the first and subsequent prescriptions from which adherence can be derived.
This cohort was linked to the HDR database, which includes information on primary diagnoses and up to five coexisting conditions, procedures performed, and dates for hospital admission and discharge. Diagnoses are classified according to the International Classification of Diseases-Ninth Revision, Clinical Modification (ICD9-CM) [16] . The Italian civil registry provides demographic information.
The study methodology adopted has been widely used to produce good epidemiology surveys [17] and [18]. The analysis was carried out in strict compliance with the national Italian regulations for full protection of the privacy rights of subjects included in the databases and in line with previous studies [17] and [18]. According to Italian law, no ethical approval is required for this type of analysis and no informed consent from patients was required.
The sample population consisted of men aged 40 yr or older who were prescribed medications for LUTS/BPH during the index period from January 1, 2004 to December 31, 2006. Only ABs and 5ARIs were considered in the analysis (ATC codes G04CA and G04CB, respectively). Other drugs were excluded because they are either not covered by the NHS or are not labeled for treatment of LUTS/BPH.
During the index period, the first prescription of a drug was considered the index date for patient inclusion. Drug adherence was measured only in patients receiving treatment for a minimum of 6 mo during the index period. Three different levels of exposure were evaluated: ≥6 mo, ≥10 mo, and ≥12 mo. Patients on treatment for more than 12 mo during the index period were followed up for 4 yr (median time). Patients who (1) stopped one of the three possible regimens (AB monotherapy, 5ARI monotherapy, or CT) for at least two consecutive months during the first year of treatment and at least 4 mo/yr during the follow-up period, or (2) switched regimen were considered as discontinued.
Patients were followed until BPH hospitalization or surgery or the last follow-up. Patients were excluded when they had a diagnosis of urethral stricture (ICD9-CM codes 598, 589.0, 598.00, 598.01, 598.1, 598.2, 598.8, and 598.9), prostate cancer (ICD9-CM codes 185, 198.82, 233.4, 236.5, 239.5, and V10.46), and/or a prescription for a gonadotropin-releasing hormone analogue and/or an antiandrogen agent in the 12 mo preceding the index day.
The Charlson comorbidity index adapted to ICD9-CM was used as a surrogate measure of comorbidities [19] . Hospital admissions were recorded in patients receiving ≥1 yr of pharmacological therapy and were considered as BPH-related when hospital records included a primary diagnosis and/or surgical procedures related to BPH. The presence of an ICD9-CM 600.xx code as the primary diagnosis without surgical procedures was considered as BPH hospitalization. Because of the lack of clear and universally accepted indications for hospitalization for BPH, we included in the analyses all hospitalization events for hematuria, urinary infection, urinary retention, bladder stones, and obstructive acute renal failure related to BPH. ICD9-CM codes 57.0, 57.91, 57.92, 60.21, 60.29, 60.3, 60.4 for primary or secondary surgical procedures for any primary diagnoses were considered as hospitalization for BPH surgery. The occurrence of hematuria (ICD9-CM 599.7), bladder stones and diverticula (ICD9-CM 592.0, 592.1, 592.9, 594.1, 563.3), bladder neck obstruction (ICD9-CM 599.7), urinary retention and obstruction (ICD9-CM 788.20, 599.6), acute and chronic renal failure (ICD9-CM 584, 585, 586), hydronephrosis (ICD9-CM 591), and urinary infection (ICD9-CM 595.0, 595.4) was also assessed to capture and characterize severity factors.
For patients with at least 12 mo of treatment, characteristics are reported using descriptive statistics. Differences between patient treatment subgroups were assessed using a standardized difference. Crude incidence rates (IRs) per 1000 patient-years and incidence rate ratios (IRRs) with 95% confidence intervals (CIs) were calculated using a Poisson regression model.
A multivariate Cox proportional hazards regression model was used to account for differences in follow-up and in baseline characteristics among groups. In all Cox models, the associations between groups and all outcomes were adjusted for covariates known to be of prognostic importance for the outcomes analyzed: age, Charlson comorbidity index, previous hospitalization for BPH, previous BPH-related surgery, pre-existing severity factors, and previous pharmacological treatment. Results are expressed as hazard ratios (HRs) and 95% CIs. Adjusted event-free survival curves were calculated using the corrected group prognosis method.
A propensity score (PS) matching analysis was conducted to check the consistency of our findings. PS is a common device to reduce bias in treatment comparisons in observational studies; we also performed a PS analysis to adjust for potential residual confounding. For this purpose, we constructed a PS using the common referent patient approach to obtain comparability among the three exposure groups. In this approach, we constructed 1:1:1 matched triplets from patients in the AB group (referent) with those in the 5ARI and CT groups [20] . Logistic regression models were first built to predict the probability (PS) to be assigned to treatment. This model included the same covariates introduced in the Cox model plus quadratic covariate terms, and a set of two-term interactions between the same covariates. Overlapping of PS between treatment and control groups was also checked, and nonoverlapping subjects were excluded. Finally, because PS methodology addresses only imbalances due to measured confounders, we also performed a sensitivity analysis to account for potential residual confounding arising from the effect of an unmeasured binary covariate [21] . All reported p values are two-tailed, and p < 0.05 was considered statistically significant.
Analyses were conducted using SAS Statistical Package release 9.3 (SAS Institute, Cary, NC, USA).
From the initial cohort of approximately 6.5 million individuals, there were 1 447 074 men aged 40 yr and older. Among these, 213 936 were prescribed at least one box of medication for LUTS/BPH ( Fig. 1 ), but only 28 273 received prescriptions for 12 mo. This group was then followed up (median 4 yr, interquartile range 2–5.3 yr) and represented our study cohort.

General characteristics are reported in Table 1 . The mean age was 70 yr (standard deviation 9.46 yr), and more than 85% of patients had a Charlson comorbidity index of 0. AB was the most frequently prescribed class (87.1%), followed by 5ARI (8.1%) and CT (4.7%); the latter was preferred in older patients (47.6% ≥75 yr old). In the PS matched analyses, groups were different only for a Charlson comorbidity index ≥3 (6.7% for 5ARI vs 4.3% for AB).
Table 1 Patient characteristics according to BPH-related treatment
| Variable | Baseline | Standardized difference (%) * | Propensity score matching | Standardized difference (%) * | Drug adherence | Standardized difference (%) * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | AB | 5ARI | CT | 5ARI vs AB | CT vs AB | Adherent patients | Discontinued patients | ||
| Patients (n) | 28 273 | 24 626 | 2309 | 1338 | 1289 | 1289 | 1289 | 12 770 | 16 003 | |||||
| Mean age, yr (SD) | 70.28 (9.46) | 69.55 (9.36) | 75.61 (8.72) | 74.49 (8.25) | 66.99 | 58.3 | 74.51 (8.03) | 74.51 (8.03) | 74.51 (8.03) | 0 | 0 | 70.15 (9.6) | 70.37 (9.34) | |
| Age, n (%) | ||||||||||||||
| 40–55 yr | 1699 (6.01) | 1641 (6.66) | 42 (1.82) | 16 (1.20) | –24.22 | –28.42 | 13 (1.01) | 13 (1.01) | 13 (1.01) | 0 | 0 | 762 (6.21) | 937 (5.86) | –1.4916 |
| 56–65 yr | 7001 (24.76) | 6579 (26.72) | 248 (10.74) | 174 (13.00) | –41.83 | –34.89 | 164 (12.72) | 164 (12.72) | 164 (12.72) | 0 | 0 | 3131 (25.52) | 3870 (24.18) | –3.0886 |
| 66–75 yr | 11120 (39.33) | 9819 (39.87) | 791 (34.26) | 510 (38.12) | –11.65 | –3.6 | 502 (38.94) | 502 (38.94) | 502 (38.94) | 0 | 0 | 4784 (38.99) | 6336 (39.59) | 1.2350 |
| 76–85 yr | 7054 (24.95) | 5555 (22.56) | 961 (41.62) | 538 (40.21) | 41.71 | 38.75 | 520 (40.34) | 520 (40.34) | 520 (40.34) | 0 | 0 | 2936 (23.93) | 4118 (25.73) | 4.1775 |
| >85 yr | 1399 (4.95) | 1032 (4.19) | 267 (11.56) | 100 (7.47) | 27.63 | 14.04 | 90 (6.98) | 90 (6.98) | 90 (6.98) | 0 | 0 | 657 (5.35) | 742 (4.64) | –3.2957 |
| Charlson score, n (%) | ||||||||||||||
| 0 | 24219 (85.66) | 21238 (86.24) | 1870 (80.99) | 1111 (83.03) | –14.23 | –8.91 | 1084 (84.10) | 1054 (81.77) | 1084 (84.10) | –6.19 | 0 | 10489 (85.48) | 13730 (85.80) | 0.8883 |
| 1–2 | 2666 (9.43) | 2223 (9.03) | 279 (12.08) | 164 (12.26) | 9.96 | 10.49 | 149 (11.56) | 149 (11.56) | 149 (11.56) | 0 | 0 | 1179 (9.61) | 1487 (9.29) | –1.0830 |
| ≥3 | 1388 (4.91) | 1165 (4.73) | 160 (6.93) | 63 (4.71) | 9.39 | –0.1 | 56 (4.34) | 86 (6.67) | 56 (4.34) | 10.21 | 0 | 602 (4.91) | 786 (4.91) | 0.0245 |
| Previous hospitalization for BPH, n (%) | 1312 (4.64) | 1048 (4.26) | 167 (7.23) | 97 (7.25) | 12.82 | 12.88 | 63 (4.89) | 63 (4.89) | 63 (4.89) | 0 | 560 (4.56) | 752 (4.70) | 0.6430 | |
| Previous BPH surgery, n (%) | 98 (0.35) | 88 (0.36) | 7 (0.30) | 3 (0.22) | –0.94 | –2.47 | 3 (0.23) | 5 (0.39) | 3 (0.23) | 2.79 | 0 | 45 (0.37) | 53 (0.33) | –0.6030 |
| Previous BPH severity factors, n (%) | 854 (3.02) | 715 (2.90) | 95 (4.11) | 44 (3.29) | 6.58 | 2.22 | 40 (3.10) | 45 (3.49) | 37 (2.87) | 2.17 | –1.37 | 392 (3.19) | 462 (2.89) | –1.7928 |
| Previous BPH-related therapy, n (%) | 16491 (58.33) | 14220 (57.74) | 1377 (59.64) | 894 (66.82) | 3.84 | 18.8 | 857 (66.49) | 857 (66.49) | 857 (66.49) | 0 | 0 | 7155 (58.31) | 9336 (58.34) | 0.0529 |
* A standardized difference >10% represents a meaningful imbalance between treatment groups for the variable considered.
BPH = benign prostatic hyperplasia; AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; SD = standard deviation.
The number of patients who received prescriptions for ≥6 mo was 97 407, which decreased to 61 298 (63%) at 10 mo and 28 273 (29%) at 12 mo ( Fig. 1 ). The proportion of patients who continued the drugs up to 10 mo was 70%, 59%, and 34% for AB, 5ARI, and CT, respectively. These rates decreased to 35%, 18%, and 9% at 12 mo ( Fig. 2 A). In men who received a prescription for at least 6 mo, the 1-yr adherence was 29%.

Patients who remained under pharmacological therapy for the entire follow-up period (median 4 yr) represented 13% of those identified in the index period. CT patients showed a higher discontinuation rate in the first 2 yr (p < 0.0001; Fig. 2 B).
During the follow-up period, there were 989 hospitalization events for BPH and 1393 for BPH surgery, with an overall rate of 9.04 (95% CI 8.49–9.62) and 12.6 (95% CI 11.96–13.28) per 1000 patient-years, respectively.
Patients on 5ARI had a reduced risk of hospitalization compared with those under AB therapy (IRR 0.39, 95% CI 0.28–0.55 vs IRR 0.18, 95% CI 0.12–0.27). CT was only associated with a reduced risk of hospitalization for BPH surgery (IRR 0.29, 95% CI 0.19–0.46) compared with AB.
Multivariate analysis confirmed that 5ARI use was associated with a reduced risk of hospitalization for BPH (HR 0.46, 95% CI 0.33–0.65) and BPH surgery (HR 0.23, 95% CI 0.15–0.35; p < 0.0001; Table 2 ). Kaplan-Meier curves showed that most of the events were observed during the first 2 yr of follow-up ( Fig. 3 A and 3B). The number needed to treat (NNT) for hospitalization for BPH as an outcome was 44 for the 5ARI group and 104 for the CT group. The NNT for hospitalization for BPH surgery was 22 for the 5ARI group and 24 for the CT group. The results of PS matched analyses, adjusted for the Charlson score, confirmed the results observed in the overall population before matching ( Table 2 ).
Table 2 Hazard ratio (95% confidence interval) according to Cox proportional hazards regression analysis for outcomes by pharmacological therapy and drug adherence
| Clinical outcome | Baseline | Propensity score matching | Drug adherence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5ARI vs AB | p value | CT vs AB | p value | 5ARI vs AB | p value | CT vs AB | p value | Discontinued vs adherent patients | p value | |
| Hospitalization for BPH |
0.46 (0.33–0.65) |
<0.0001 | 0.94 (0.68–1.31) |
0.7307 | 0.39 (0.21–0.70) |
0.0018 | 0.85 (0.52–1.40) |
0.5296 | 1.65 (1.43–1.89) |
<0.0001 |
| Hospitalization for BPH surgery |
0.23 (0.15–0.35) |
<0.0001 | 0.36 (0.23–0.57) |
<0.0001 | 0.23 (0.12–0.45) |
<0.0001 | 0.35 (0.19–0.64) |
0.0006 | 2.80 (2.59–3.03) |
<0.0001 |
AB = alpha blocker monotherapy; CT = combination therapy; 5ARI = 5-alpha reductase inhibitor monotherapy; BPH = benign prostatic hyperplasia.

Sensitivity analysis showed that to modify the results, an unmeasured confounder should have a hypothetical HR of at least 3.75 and 2 for 5ARI and CT, respectively, and an asymmetric distribution between the treatment groups (both 5ARI and CT vs AB) of at least 90% for BPH surgery. For nonsurgical hospitalizations, the results related to the comparison between 5ARI and AB should be modified with an unmeasured confounder with HR of at least 1.75 and an asymmetric distribution between the two groups of at least 80%.
During the follow-up period, only 12 270 patients (43.4%) continued their pharmacological therapy ( Table 1 ). The therapy discontinuation rate was significantly higher for CT, with a standardized difference of 19.87% between discontinued and adherent patients (data not shown).
In multivariate analysis, drug discontinuation was an independent risk factor for BPH (HR 1.65, 95% CI 1.43–1.89) and BPH surgery regardless of therapeutic group (HR 2.80, 95% CI 2.59–3.03; p < 0.0001; Table 2 ).
BPH represents a major public health issue because of its high prevalence, progressive nature, and associated costs [22], [23], [24], and [25]. Current guidelines recommend the use of AB and 5ARI alone or in combination for the treatment of LUTS/BPH [5] and [6]. However, a natural gap exists between guidelines and actual clinical practice [10] . Low adherence to prescribed medications is a well-known issue for chronic diseases [13] . Little information is available on drug prescription and adherence and the impact on clinical outcome for pharmacological treatment of LUTS/BPH. Our data demonstrate that adherence to BPH pharmacological therapy is low and can influence BPH-related hospitalization rates. Moreover, the results confirm that 5ARI use is associated with a reduced likelihood of hospitalization for BPH and BPH surgery in real life.
Using a similar approach for patients newly diagnosed with LUTS/BPH, Verhamme et al [12] found that the average adherence differed slightly between drugs (67% AB, 73% 5ARI, 71% CT), with 26% of treated patients discontinuing their treatment early. Nichol et al [26] reported that in a total population of 2640 men, there was 40% adherence for any LUTS/BPH medication (mean follow-up 2 yr) and that discontinuation was a strong predictor of BPH surgery (odds ratio 4.17, 95% CI 2.56–6.79; p < 0.0001). We have to consider that randomized trials have shown that the benefit of 5ARIs either alone or in combination with AB regarding the risk of urinary retention and surgery appears after a minimum of 1 yr. In summary, there is no rationale for short-term 5ARI use.
We found very poor 1-yr drug adherence (overall 29%) in a large cohort of patients pharmacologically treated for LUTS/BPH, with substantial variations among drug classes. Moreover, we found that prolonged exposure to 5ARI and adherence to the prescribed treatment regimen were each significantly associated with a lower risk of BPH-related hospitalization and surgery, which is plausible and as expected.
In the LUTS/BPH population, the decision to adhere to pharmacological treatment is primarily based on patient perception of discomfort and inconvenience, and possibly on patient expectations. BPH is a unique condition that is no longer life-threatening, despite being potentially progressive. Thus, the patient plays a major role in the decision to initiate and continue treatment [27] .
We can speculate that the high discontinuation rate observed in our record-linkage analysis may be attributable to a combination of drug side effects and patient expectations, although an incorrect diagnosis (prostatitis, overactive bladder, interstitial cystitis) at baseline cannot be excluded. Experience in other fields suggests that the majority of patients stop taking drugs because the perceived improvement is lower than expected. Although patients also switch to new medications, it has been shown that the development of coping strategies and side effects also play a role [28] . Some patients may stop taking a drug because of deterioration in their sexual function, and others may not understand that clinical improvement is observed after a minimum of 3–6 mo. In some cases, the symptomatic improvement perceived by patients after a few months of treatment may lead them to stop taking the medication because the bothersome condition has disappeared. Proper patient counseling on the long-term effect of 5ARIs on disease progression and risk of retention/surgery may be of importance to improve drug adherence. In this study, the CT group represented only 4.7% of the whole cohort. This reflects either the attitude of physicians or low patient adherence to this regimen, and seems to be one of the most striking discrepancies between guidelines and actual practice. Proper health economic analysis of the impact of drug adherence on the costs of managing BPH is necessary. We observed progressive discontinuation of pharmacological treatment regardless of the therapy group. This suggests that besides side effects and lack of efficacy, scarce and suboptimal awareness of disease progression is probably another major reason for discontinuation. In this respect, interventions focused on proper patient counseling to improve adherence to BPH medications are of primary importance.
In our study, 1393 hospitalizations for surgical reasons (12.6 per 1000 patient-years) and 989 for nonsurgical reasons (9.04 per 1000 patient-years) were recorded during follow-up. Our findings are in accordance with previous data [12] showing that a progressive reduction in BPH-related hospitalizations during the last decade of life can be achieved in adherent patients. Multivariate analysis revealed that 5ARI and CT were associated with an independent reduced likelihood of hospitalization for BPH surgery, as already reported by others in different experimental settings [7], [8], and [29]. This is not surprising because trials have shown that even 5ARIs can modify BPH progression in the first year of treatment [7] .
Although studies based on administrative data sets are constantly increasing, all the inherent limitations should be acknowledged [30] . Administrative databases studies cannot be considered efficacy studies and do not include clinical variables or patient-reported outcomes because they are developed mainly for billing purposes [14] . However, administrative data, although imperfect, continue to play an important role in health research and their use should be promoted because of their low cost, wide availability, and ability to span multiple years and health care settings [14] and [30].
The available data do not allow assessment of: (1) whether prescribing was by urologists or general practitioners; (2) whether changes in prescriptions were related to clinical variations or subjective decisions; (3) how exposure time to treatment was related to filled prescriptions, which may not faithfully measure drug intake; (4) information on symptomatic patients who are not taking treatment; and (5) whether the presumed diagnosis of LUTS/BPH was confirmed during the index period.
Another severe limitation is the imbalance between regimens: the CT group represents 4.7% of the whole cohort. The small size of this group compromised the value of further subanalyses but reflects the attitude of physicians. The results of the current analysis are obviously specific to the Italian situation and driven by local health policies, the social environment, and health care costs. Moreover, in the discontinued group we considered all patients who abandoned, changed, switched, or interrupted treatment.
Even considering its limitations, this is one of the few studies reporting on long-term drug adherence and its clinical value using administrative databases. Specifically, there is little evidence available on the impact of long-term treatment and drug adherence on BPH clinical progression in real life, although further evaluations are designed.
Our findings suggest that overall adherence to BPH pharmacological therapy is low, and that persistence with pharmacological treatment is associated with a lower rate of hospitalization for BPH-related reasons. 5ARI use is associated with an independent reduced likelihood of hospitalization for BPH.
Author contributions: Luca Cindolo had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Cindolo, Romero.
Acquisition of data: Romero.
Analysis and interpretation of data: Fanizza, Pirozzi, Cindolo.
Drafting of the manuscript: Cindolo, Pirozzi, De Nunzio.
Critical revision of the manuscript for important intellectual content: Autorino, Tubaro, Schips.
Statistical analysis: Fanizza.
Obtaining funding: Cindolo.
Administrative, technical, or material support: Pirozzi.
Supervision: Schips.
Other (specify): None.
Financial disclosures: Luca Cindolo certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Luca Cindolo conducts surgical tutorship for American Medical Systems and has received honoraria from GlaxoSmithKline for presentations. Andrea Tubaro is a consultant for and has received research grants from Allergan and Astellas, is an investigator and paid speaker for American Medical Systems, gives presentations for Ferring, GlaxoSmithKline, and Pfizer, is a consultant for Bayer, and is a consultant and investigator for GlaxoSmithKline. The remaining authors have nothing to disclose.
Funding/Support and role of the sponsor: GlaxoSmithKline provided an unrestricted grant for this research. The sponsor played no role in the study.
Acknowledgments: We thank Kimberlee Ann Manzi for reviewing the linguistic style of the manuscript.
Data sharing: A full data set and the statistical code used for analysis are available from the authors on request. These data can be used only for replication of the analyses published in this paper or for private study. Express written permission must be sought from the authors for any other data use.