Hypothesis / aims of study
Urinary tract infections (UTI) are one of the most common problems in patients with neurogenic low urinary tract dysfunction (NLUTD). Reorganization of urinary microbiome in neurogenic bladder plays the important role in UTIs. However, we still do not know how the different types of therapy, particularly, botulinum toxin injections, change the microbiome. The aim was to assess the urinary microbiome in neurogenic bladder patients with high recurrence of UTIs before and after botulinum toxin therapy using 16S rRNA gene (16S rDNA) sequencing and metaproteomics.
Study design, materials and methods
Following written consent, urine samples were obtained from 6 patients with NLUTD due to different neurogenic diseases before intradetrusor botulinum toxin injections and 6 weeks after. Recruited patients had NLUTD for many years, performing intermittent catheterization and suffering from often exacerbations of UTIs. They have undergone an urodynamic study using Triton (Laborie) machine with air-charged catheters. The presence of high-pressure detrusor overactivity had been proven in all patients. Previous therapy with anticholinergics had had no effect.
Injections of 200 Units (UI) of onabotulinumtoxin A (Botox, Allergan) where performed in operation room under intravenous aesthesia directly in the detrusor in 20 points (10UI/point) with cystoscope and end endoscopic needle (injeTAK®).
Urine samples where investigated using standart urinalysis, enhanced quantitative urine culture (EQUC) method and 16S rRNA gene (16S rDNA) sequencing.
Results
Patients with neurogenic detrusor overactivity had been treated with 200 UI of onabotulinumtoxin A. All had a clinical and urodynamic improvement confirmed by increasing of a bladder cystometric capacity by 187.3±79.51 ml, bladder volume at the time of first involuntary detrusor contraction by 285.5±94.8 ml and decreasing of maximal detrusor pressure during involuntary detrusor contraction by 34.8±25.0 cm H20.
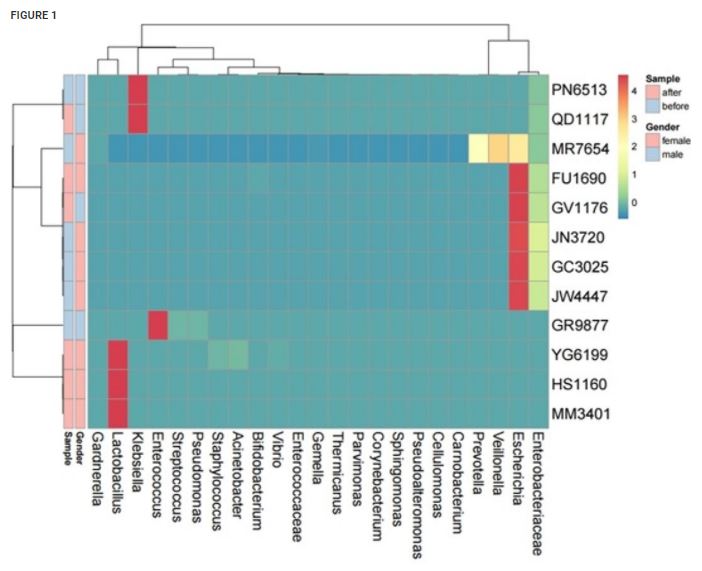
The results of the urine culture correlated with 16S rRNA sequencing results, but the greater amount of bacterial taxa were detected by metagenomics sequencing. Analysis of the bacterial community in urine samples revealed between 2 and 21 species-level reads per individual. All patients before botulinum therapy demonstrated abnormal urinary microbiome with predominance of Enterobacteriales. Female patients were more likely to have predominantly lactobacillus in urine after botox injections than before. In three cases in females, microbiome had been changed after therapy from Enterobacteriaceae to Lactobacillales. In one female and two males, no principal changes had happened.
The clinical follow up for six month after botulinum toxin injections showed that in all cases frequency of UTIs has decreased. Male patients had no UTIs for half a year. Two women with positive changes in microbiome also had no UTIs and one girl had one episode of UTI. Female patient with abnormal microflora had two episodes – maximal count of UTIs among all patients.
Interpretation of results
Patients with NLUTD have modified microbiome with the prevalence of Enterobacterales. The study showed that in females improving in urodynamic parameters after botulinum toxin injections may be associated with changing in the urine microbiome towards a normal flora. It expands our understandig of the UTIs development in patients with neurogenic bladder and the protective effect of the botulinum toxin therapy.
Concluding message
This is the first report comparing in pilot study the urine microbiome in neurogenic bladder patients before and after botulinum toxin injections into the bladder wall. The results are restricted by the small number of participants because of the high cost of the 16S rRNA sequencing. However, the promising observation about the influence of botulinum toxin therapy on urinary microbiome was obtained.
Disclosures
Funding No Clinical Trial No Subjects Human Ethics Committee Local Ethics Committee of Ural State Medical University Helsinki Yes Informed Consent Yes