Background
The nocturnal intermittent hypoxia caused by obstructive sleep apnea syndrome (OSAS) can provoke the sympathetic nervous activity (SNA). Salivary alpha-amylase (sAA) is a sensitive, non-invasive biomarker for reflecting the SNA, and a useful marker for pediatric OSAS subjects. Adenotonsillar hypertrophy (ATH) is the most commonly identified risk factor in OSAS childhood, therefore, several studies showed that the adenotonsillectomy (T&A) may alleviate nocturnal enuresis (NE) in children with OSAS.
Objective
The present study was to investigate the effect of T&A on NE, the change of sAA value in ATH and OSAS children, with/without NE, and with/without the operation.
Study design
37 children (Group A) were admitted for ATH and NE. The saliva samples were taken before and after polysomnography for the measure of sAA. After the T&A, the children were followed-up for 1 year. 35 OSAS children with NE but no T&A were as a NE watchful-waiting group (Group B), 32 subjects without OSAS or NE were as non-OSAS control (Group C), 42 cases who underwent T&A but did not have NE were admitted to evaluate the SNA (Group D). Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points.
Results
The observational results in the present study showed a significant rate of the disappearance of NE 1 month after the T&A and had an almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and the SNA in children with OSAS (Figure).
Discussion
Little research has previously focused on the relationship between childhood OSAS and the SNA. No data are currently available regarding comparisons of sAA levels before and after the T&A in children with OSAS and enuresis. Our findings in this present study showed that there was a resolution or decrease in enuresis events and drops in sAA levels following T&A, which were consistent with earlier study. However, there was no significant difference in the urinary catecholamine levels was found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters.
Conclusions
T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. sAA might be associated with instability of ANS by OSAS and have a consistent relationship with the apnea-hypopnea index. Our studying aims had been met.
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
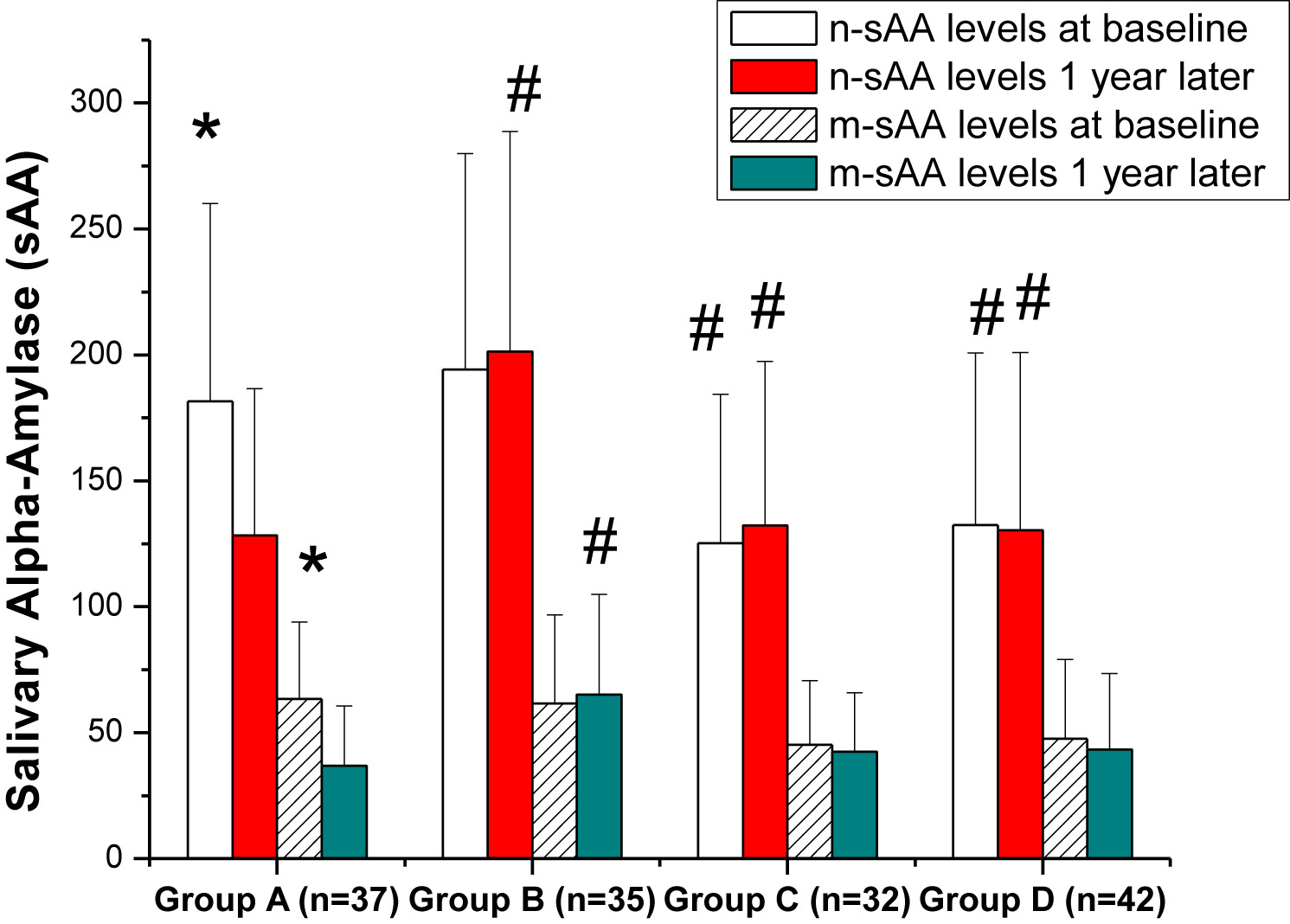
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
Obstructive sleep apnea syndrome (OSAS) is a type of sleep-disordered breathing that is characterized by intermittent, partial or complete upper airway obstruction [1] and [2]. A peak incidence of this syndrome is in childhood at 2–8 years of age [3], [4], and [5]. The most commonly identified risk factor is adenotonsillar hypertrophy (ATH) [6]; therefore, the definitive treatment is adenotonsillectomy (T&A) [2], [6], [7], and [8]. Numerous adverse health outcomes, including cognitive and behavioral deficits, are associated with OSAS [2]. Compared with adult cases, nocturnal enuresis (NE) is a relatively common finding in children [9]; it has been reported that as many as 47% of children with OSAS have NE [9]. Several studies have investigated NE in children with upper airway obstruction [9], [10], [11], and [12] and the effect of T&A [11], [12], [13], and [14]. Some reports have agreed that there is a resolution or decrease in enuresis events following T&A.
The nocturnal intermittent hypoxia caused by OSAS can activate oxidative stress and provoke sympathetic nervous activity (SNA) [15] and [16]. The elevated sympathetic tone is probably attributed to the increased risk of neurocognitive and cardiovascular events, and endocrine and metabolic comorbidity [17]. However, little research has previously focused on the relationship between childhood sleep-disordered breathing and SNA. Regulated by the sympathetic nervous system, salivary alpha-amylase (sAA) has been recommended as a sensitive, non-invasive biomarker for physiological stress-related changes in the body that reflect the SNA [18]. A growing body of resulting literature has consistently shown the validity and reliability of this emerging parameter, and been a useful marker for OSAS with pediatric subjects [19]. Furthermore, sampling for sAA is easy and pain-free to collect, and optimal for multiple sampling in children [19].
By understanding that the increasing SNA induced by hypoxia during OSAS could be represented by sAA, and that T&A may eliminate enuresis events in children with OSAS, it was speculated that the estimation of sAA might predict the presence and severity of SNA in children with NE who are diagnosed with OSAS. However, no data are currently available regarding comparisons of sAA levels before and after T&A in children with OSAS and enuresis.
Therefore, the present study compared the frequency of NE before and after T&A operation, with or without the procedure, in pediatric OSAS subjects with ATH. It also explored the change of sAA value in ATH and OSAS children, with or without NE, and with or without the operation.
A total of 146 consecutive children, with a range of 5–9 years of age, who were examined at the sleep laboratory of the present institution between January 2011 and May 2015 were prospectively enrolled in this study. The Medical Ethics Committee of the Medical School at Wuhan University had approved the protocol for this study. Written informed consent was obtained from their parents.
Eligible children in Group A were those with OSAS caused by ATH and NE (at least 1 episode per week) with snoring or OSAS without prolonged oxyhemoglobin desaturation. They were first screened by a detailed clinical history and sleep questionnaire completed by each child's parents, a complete physical examination by a pediatric pulmonologist or an otolaryngologist, and confirmed by nocturnal polysomnography. Their family members consented to the T&A operation, the pre-operative polysomnography, and agreed to participate in the postoperative phase of this study.
Exclusion criteria included severe polysomnography findings with an apnea-hypopnea index ≥30 events/hour, or an obstructive apnea index ≥20 events/hour, or arterial oxyhemoglobin saturation of <90% for ≥2% of the total sleep time. All participants in the study received a full evaluation for secondary causes of enuresis. Children with enuresis caused by other systemic chronic medical conditions or those pharmacologically treated for NE were excluded from the study.
In the standard sleep questionnaire, parents were asked whether their child currently suffered from monosymptomatic or nonmonosymptomatic enuresis, and whether they had a dry period of at least 6 months; however, no daytime urinary symptoms were included. A total of 37 children met the inclusion criteria (OSAS + NE + T&A, Group A). To compare the frequency of NE and the change of sAA level, 35 OSAS children with ATH and NE but no T&A (their parents did not agree to the operation) were an NE watchful-waiting group (Group B, 13 cases with frequent NE and 22 with occasional NE). Another 32 subjects without OSAS (obstructive apnea-hypopnea index <2 or obstructive apnea index <1) or NE were a non-OSAS control (Group C). A further 42 cases who underwent T&A for simple ATH but did not have NE were also admitted into this study to assess the SNA (Group D). However, the pediatric or otolaryngologic interviewers did not know which group the subjects belonged to.
Each child was taken to the sleep laboratory and overnight polysomnography was performed according to the standards of the American Thoracic Society [20]. The Sandman® Elite sleep diagnostic system (Puritan Bennett, Pleasanton, CA, USA) was used to record and analyze the variables. Sleep staging and arousals were scored according to the criteria of Rechtschaffen and Kales [21] and the American Academy of Sleep Medicine criteria [22] and [23]. The same investigator performed all scoring. Obstructive apneic and hypopneic events were scored according to the recommended pediatric criteria [22] and [23]. Obstructive apnea was defined as paradoxical breathing for ≥2 respiratory cycles, with complete cessation of inspiratory airflow. Hypopnea was defined as a reduction in the amplitude of respiratory effort of ≥50% from the sleeping baseline level, or a discernible reduction that did not reach the above criteria but was associated with at least a 3% decrease in arterial oxyhemoglobin saturation or an arousal. The apnea-hypopnea index was calculated as the average number of apneas and hypopneas per hour of sleep. OSAS was defined as an obstructive apnea-hypopnea index of ≥2 events/hour or an obstructive apnea index ≥1 event/hour [6] and [24].
Because of an obvious diurnal pattern of sAA activity [25], the saliva samples were collected in special tubes around 22:00 before polysomnography and again the next morning within 30 min of polysomnography ending. The sAA concentration was measured according to protocol by use of a commercially available kinetic reaction assay (ZheJiang Ikon Biotechnology Co., Ltd, Shaoxing, China).
In order to effectively collect saliva, the children were prevented from eating a meal for at least 1 h before gathering the samples. Moreover, children were told not to eat or drink dairy products and sugary foods on polysomnography day, and their mouths were rinsed with water 10 min before saliva sampling.
For the measurement of urinary excretion of epinephrine and norepinephrine, overnight urine from 21:00 to 07:00 during the sleep study was collected using containers acidified with 10 ml of 6 M hydrochloric acid. Catecholamines were analyzed using high-performance liquid chromatography.
The otolaryngologist made the final decision on the indication for the T&A, and ENT specialists performed the surgical procedures.
Follow-up included evaluations for NE, sAA and urinary catecholamine after the T&A or at the equivalent time points. All the participants were repeatedly assessed for their levels of sAA and urinary catecholamine for 12 months. However, overnight polysomnography was re-performed only in the OSAS groups, because most parents of non-OSAS groups did not agree the second tests for their children. Clinical NE assessments were performed by repeating the original standard structured telephone questionnaire adding a parental assessment of NE frequency at three time-points: (1) 1 month after the T&A; (2) 3 months after the T&A; and (3) 1 year later. The frequency of NE was graded according to Brooks and Topol's criteria [9]: frequent (≥3 times/week), occasional (1–2 times/week), rare (<1 time/week), or never.
All quantitative variables (polysomnography parameters, sAA values and levels of catecholamine excretion) were analyzed for normal distribution using box plots and were reported as means ± SEM. Two-way repeated measures analysis of variance were used to compare all variables at baseline or at 1 year. The four groups were compared if the overall F-value was significant (P < 0.05). Statistical comparisons between ‘frequent’ or ‘occasional’ NE before and after the operation, NE in boys and girls, and other pairwise multiple comparisons were made using the Least Significant Difference method. Differences in characteristics between participants with and without NE symptoms or OSAS or T&A were compared by using t-tests or a nonparametric equivalent for continuous variables, and Chi-squared tests for dichotomous variables. Spearman correlation coefficients were used to assess the relationship between the sAA or catecholamine measurements and the polysomnography parameters in OSAS patients. A P-value of <0.05 was regarded as statistically significant.
A total of 146 subjects, aged 5–9 years, were enrolled into the study, and were divided into four groups. The demographic data and baseline polysomnography findings were as listed in Table 1. Number, gender, age and height were not significantly different between the groups (P > 0.05). The body mass index (BMI) and some polysomnography findings, including the apnea-hypopnea index, and oxygen desaturation index in Group A or B were significantly higher than in Group C or D (P < 0.05); but the lowest oxygen saturation in the OSAS groups was significantly lower than in non-OSAS groups (P < 0.05).
Table 1
General characteristics and PSG findings at baseline in four groups.
| Parameters | Groups | |||
|---|---|---|---|---|
| A | B | C | D | |
| Number | 37 | 35 | 32 | 42 |
| Boy/Girl (n) | 26/11 | 25/10 | 20/12 | 30/12 |
| Age (years) | 7.3 ± 1.6 | 7.1 ± 1.9 | 7.4 ± 1.8 | 7.2 ± 1.7 |
| Weight (kg) | 28.1 ± 9.7 | 28.6 ± 11.3 | 26.5 ± 10.4 | 27.3 ± 11.6 |
| Height (cm) | 112.7 ± 10.5 | 114.3 ± 11.3 | 113.8 ± 9.4 | 114.2 ± 10.3 |
| BMI (kg/m2) | 22.3 ± 6.8 | 21.9 ± 9.2 | 20.6 ± 7.8* | 20.9 ± 8.6* |
| AHI (events/hour) | 8.3 ± 1.9 | 7.8 ± 2.7 | 0.6 ± 0.2# | 1.1 ± 0.4# |
| SaO2 nadir (%) | 87.2 ± 0.5 | 86.5 ± 1.1 | 94.3 ± 1.4* | 93.8 ± 1.6* |
| ODI (events/hour) | 3.1 ± 0.4 | 3.2 ± 0.9 | 0.1 ± 0.4# | 0.4 ± 0.3# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index. Compared with group A or B, *P < 0.05, #P < 0.01.
All children in Group A were found to have an obstructive apnea-hypopnea index ≥2 and obstructive apnea index ≥1, with a mean apnea-hypopnea index of 8.3 ± 1.9 events/hour (Table 1). However, there was no significant difference in the presence of NE between boys and girls (P > 0.05). The relationship between baseline apnea-hypopnea index and frequency of NE in all children undergoing T&A was analyzed (Table 2). Children with NE frequencies of ‘frequent’ and ‘occasional’ had an average apnea-hypopnea index of 8.6 ± 2.0 events/hour and 8.1 ± 2.3 events/hour, respectively. This difference was not statistically significant (P > 0.05). Meanwhile, there were no significant differences in polysomnography parameters among different frequencies of NE in children with OSAS (P > 0.05).
Table 2
Population characteristics and PSG findings before T&A in Group A.
| Parameters | Enuresis before T&A | |||
|---|---|---|---|---|
| Frequently (>3/W) | Sometimes (1–2/W) | Rarely (<1/W) | Never 0 |
|
| Number | 12 | 25* | ||
| Boy/Girl (n) | 9/3 | 17/8† | 0/0 | 0/0 |
| Age (years) | 7.4 ± 1.8 | 7.2 ± 1.6† | NA | NA |
| Weight (kg) | 28.6 ± 10.4 | 27.9 ± 9.9† | NA | NA |
| Height (cm) | 112.5 ± 10.3 | 112.8 ± 11.1† | NA | NA |
| BMI (kg/m2) | 21.9 ± 7.0 | 22.5 ± 8.2† | NA | NA |
| AHI (events/hour) | 8.6 ± 2.0 | 8.1 ± 2.3† | NA | NA |
| SaO2 nadir (%) | 86.7 ± 1.2 | 87.5 ± 1.1† | NA | NA |
| ODI (events/hour) | 3.2 ± 0.5 | 3.1 ± 0.8† | NA | NA |
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05, *P < 0.05.
NA, no data; BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
All of the children in Group A underwent T&A. One month after the procedure, 81.1% (30/37) of the children reported a decrease in NE severity. The NE totally disappeared in 64.9% (24/37) of children within 1 month, and all the rest had a postoperative improvement in NE. By the time of the second follow-up, another 18.9% (7/37) reported that NE had disappeared. The remaining four cases eliminated their NE throughout the remaining time of the 1-year follow-up. Only two children had rare NE by the end of the follow-up (Table 3). The disappearance rate of NE 1 year after T&A in Group A was significantly higher than the reported spontaneous resolution rate in Group B (94.6% vs 17.1%, P < 0.001) (Table 4).
Table 3
Frequency of NE after T&A in Group A.
| Enuresis frequency | Frequent | Occasional | Rare | Never |
|---|---|---|---|---|
| At time of polysomnography | 12 | 25 | ||
| After T&A | ||||
| 1 month | 2* | 5* | 6 | 24# |
| 3 months | 0* | 2* | 4 | 31# |
| 1 year | 0* | 0* | 2 | 35# |
Compared with the NE frequencies on PSG, *P < 0.05, #P < 0.01.
Table 4
Frequency of NE after T&A or at the equivalent time points in four groups.
| Time after T&A or at the equivalent point | Group | |||
|---|---|---|---|---|
| A (n = 37) | B (n = 35) | C (n = 32) | D (n = 42) | |
| 1 month | 13 | 34* | ||
| 3 months | 6 | 32# | ||
| 1 year | 2 | 29# | ||
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
Group C: no OSAS or NE (normal control).
Group D: ATH + T&A (no OSAS or NE).
Compared with group A, *P < 0.05, #P < 0.01.
The results are shown in Fig. 1. In sAA measurements, there were no significant differences between Group A and B or Group C and D. However, comparisons between the OSAS groups and non-OSAS groups showed a significant difference in night sAA (n-sAA) and morning sAA (m-sAA); both were remarkably higher in the OSAS groups than in the non-OSAS groups (P < 0.05). Urinary catecholamine (norepinephrine and epinephrine) levels in the four groups had the same phenomena (P < 0.05). Table 5 shows the measurement of sAA and urinary catecholamine according to NE frequency. However, there were no significant differences in levels of sAA and catecholamine among different frequencies of NE in children with OSAS (P > 0.05).

Figure 1
Baseline levels of (a) sAA and (b) urinary catecholamine in four groups. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A or B.
Table 5
sAA and urinary catecholamine levels according to NE frequency in Group A and B.
| Parameters | Frequency of nocturnal enuresis | |
|---|---|---|
| Frequent (n = 25) | Occasional (n = 47) | |
| Amylase | ||
| n-sAA | 190.2 ± 80.8 | 185.6 ± 76.4† |
| m-sAA | 62.4 ± 36.3 | 62.6 ± 34.6† |
| UNE (μg/night) | 89.2 ± 22.6 | 88.8 ± 21.7† |
| UE (μg/night) | 5.4 ± 2.2 | 5.6 ± 2.1† |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Compared with the children with NE frequencies of ‘frequent’, †P > 0.05.
Fig. 1 shows that there were significant differences between the OSAS groups and non-OSAS groups for sAA and urinary catecholamine measurements. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index (P < 0.05, Table 6). However, no sAA measurements were associated with the lowest oxygen saturation (P > 0.05). Furthermore, the urinary catecholamine did not have relationship with polysomnography parameters.
Table 6
Relationship of sAA to PSG parameters in OSAS patients (Spearman correlation coefficients).
| Parameters | AHI | ODI | SaO2 nadir | |||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| n-sAA | 0.39 | 0.017 | 0.41 | 0.012 | −0.16 | 0.236 |
| m-sAA | 0.36 | 0.015 | 0.44 | 0.007 | −0.18 | 0.232 |
| UNE | 0.22 | 0.16 | 0.09 | 0.611 | −0.28 | 0.361 |
| UE | 0.11 | 0.21 | 0.13 | 0.241 | −0.26 | 0.327 |
n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine.
Polysomnography follow-ups 1 year later in Group A and B are illustrated in Table 7. The values of apnea-hypopnea index, lowest oxygen saturation and oxygen desaturation index in Group A reached the normal range. Nevertheless, compared with 1 year previously, there were no significant differences in the polysomnography parameters in Group B. On the other hand, before the T&A operation, the sAA (n-sAA, m-sSS) and urinary catecholamine (norepinephrine, epinephrine) were significantly higher in Group A or B than in Group C or D. However, 1 year after the operation, sAA and urinary catecholamine in Group B were much higher than in Group A. Both the levels of sAA and urinary catecholamine in Group A decreased to the levels in non-OSAS groups (Fig. 2).
Table 7
General characteristics and PSG findings 1 year after operation or at the equivalent point.
| Parameters | Group A | Group B |
|---|---|---|
| Number | 37 | 35 |
| Weight (kg) | 32.3 ± 10.1 | 32.6 ± 11.8† |
| Height (cm) | 116.6 ± 11.3 | 117.3 ± 9.8† |
| BMI (kg/m2) | 22.8 ± 5.6 | 23.24 ± 9.4† |
| AHI (events/hour) | 1.3 ± 0.4 | 7.1 ± 2.3# |
| SaO2 nadir (%) | 93.6 ± 2.1 | 85.7 ± 2.1* |
| ODI (events/hour) | 0.5 ± 0.3 | 3.4 ± 0.8# |
Group A: OSAS + NE + T&A.
Group B: OSAS + NE, no T&A.
BMI, body mass index; AHI, apnea hypopnea index; ODI, oxygen desaturation index.
Compared with group A, *P < 0.05, #P<0.01, †P > 0.05.

Figure 2
Levels of (a) sAA and (b) urinary catecholamine in four groups 1 year later. Group A: OSAS + NE + T&A. Group B: OSAS + NE, no T&A. Group C: no OSAS or NE (normal control). Group D: ATH + T&A (no OSAS or NE). n-sAA, the sAA level at night; m-sAA, the sAA level in the morning; UNE, urinary norepinephrine; UE, urinary epinephrine. *P < 0.05, compared with Group A, C or D.
The observational data in the present study showed a significant rate of disappearance of NE 1 month after the T&A, and that there was almost complete resolution 1 year later. OSAS may irritate oxidative stress and increase SNA in pediatric subjects, which is reflected by increased levels of sAA and urinary catecholamine, while the T&A can decrease enuresis and SNA in children with OSAS.
OSAS is an important cause of secondary NE in children. It has been reported that there is a higher occurrence of NE (8–47%) [9] among children with OSAS than in otherwise healthy pediatric patients [26]. Brooks and Topol [9] reported that children with OSAS (respiratory disturbance index >1 events/hour) had a significantly higher prevalence of NE (47%) than those with a respiratory disturbance index of ≤1.
T&A is the primary treatment for childhood OSAS, because the most commonly identified risk factor is ATH. In sleep apnea confirmed by polysomnography, T&A relieves the problem in close to 80% of children [27]. A large randomized trial of T&A for childhood sleep apnea [6] provided evidence for the beneficial effects of early T&A on children with OSAS; however, the incidence of NE was not reported and the effect of T&A on NE was not assessed in that study. Some case reports and case series have tried to assess NE in children with OSAS [9], [10], [11], and [13] and the effect of T&A on NE [10], [11], [12], and [14]. Most of those results showed that the NE was resolved or significantly reduced after OSAS treatment. Weider et al. [10] investigated a total of 115 children with NE and OSAS and the effectiveness of T&A, and reported that there was a 77% reduction in NE 5 months after the surgery. The present results show that there was 94.6% resolution rate of NE within 1 year after the T&A, which was significantly higher than the reported spontaneous resolution in OSAS children with ATH but no T&A.
The mechanism of NE in children with OSAS is unclear. Elevated atrial natriuretic peptide and decreased antidiuretic hormone may contribute to nocturnal urinary output and polyuria in adults with OSAS [28], [29], and [30]. Increased intrathoracic negative pressure may result in elevated preload and atrial volume, which stimulates the production of atrial natriuretic peptide [28]. Therefore, treating OSAS using T&A might eventually result in a reduction of atrial volume and the secretion of atrial natriuretic peptide, which in turn, can decrease nocturnal urinary output [31].
Intermittent hypoxia and sleep fragmentation related to sleep apnea might induce chronic SNA activation, which might alter chemoreflexes and baroreflexes and result in sympathetic activation [32] and [33]. Regulated by the sympathetic nervous system, sAA has been recommended as a distinctive indicator of SNA and hypothalamus-pituitary-adrenal gland axis activity [18]. Due to being well related to polysomnography parameters, such as the apnea-hypopnea index and oxygen desaturation index, it has been used as a non-invasive biomarker of OSAS in children [34]. The estimation of sAA might predict the presence and severity of sleep-disordered breathing in children with OSAS [19]. The sAA has characteristic diurnal patterns [35] and [36]; however, collection of a morning (waking) and an evening sample was sufficient to accurately characterize the diurnal slope [37].
For evaluating SNA diurnal pattern, in the present study, the levels of sAA at night before sleep and in the early morning after waking were measured to evaluate the relationships with OSAS and NE. These results showed that there were statistically significant differences in absolute values of n-sAA, m-sAA and urinary catecholamine between the OSAS groups and non-OSAS groups, with or without NE. However, there were no significant differences in the both measurements among the OSAS groups at different degrees of NE. Further attempts have been made in this study to explore the correlation between the sAA or urinary catecholamine and polysomnography parameters or T&A, with the aim of determining the influence of T&A to sAA. The results demonstrated that the T&A reduced the apnea-hypopnea index or oxygen desaturation index, the sAA and urinary catecholamine. The n-sAA and m-sAA were positively associated with the apnea-hypopnea index and oxygen desaturation index, whereas no sAA measurements were associated with the NE and the lowest oxygen saturation. These findings were consistent with an earlier study [19]. On the other hand, it was described in the present study that there were no significant differences in the urinary catecholamine levels found between OSAS groups with or without NE. Furthermore, there was no correlation between the urinary catecholamine and polysomnography parameters. The finding was inconsistent with previous results by Kelly and colleagues [38]. They found that worsening OSA (total apnea-hypopnea index and oxygen desaturation) in obese pubertal children were associated with increasing urinary normetanephrine and norepinephrine. However, the differences in results may be related to non-obese children who had a lower apnea-hypopnea index in the present study.
Above all, due to collecting being easy, non-invasive and pain-free, sAA it is suitable for multiple sampling in children to evaluate the therapeutic outcome and the instability of SNA associated with OSAS. Of course, it does not imply that sAA can replace the role of polysomnography in the diagnosis of pediatric sleep-disordered breathing.
A major limitation of the present study was that overnight polysomnographys were not re-performed in all enrolled children, as most parents did not agree the second tests of their children. So, the relationship between SNA and T&A cannot be further evaluated. However, this limitation did not influence the assessment of outcomes.
According to the present findings, SNA is elevated and NE is a distinct symptom in children with OSAS. T&A has a favorable therapeutic effect on NE and may decrease SNA in children with OSAS. As a distinctive indicator of sympathetic activity, sAA might be associated with instability of SNA by OSAS and have a consistent relationship with the apnea-hypopnea index.
This was not an industry-supported study. Support for this study was provided by the grants from the National Natural Science Foundation of China (No. 81370181). The authors have declared that there are no conflicts of interest. Hao Ding contributed equally to Mengmei Wang.
This work was supported by the grants from the National Natural Science Foundation of China (No. 81370181).
There is accumulating evidence that at least a number of children with enuresissuffer from sleep-disordered breathing. Nocturnal hypoxia can influence renalfunction, bladder function and arousal. Treatment with respiratory support,adenotonsillectomy (A&T), or even orthodontic treatment has been shown tolead to resolution of enuresis in some but not all children.This paper concerns the autonomous nervous system as part of these processes.The authors identified four groups of children, 37 children with sleep disorderedbreathing and enuresis that underwent A&T, 35 children with both diagnoseswho were not treated with A&T, 32 healthy controls and 42 children whounderwent A&T but did not suffer from enuresis. The biochemicaldeterminations used to evaluate the autonomic activity was saliva alpha-amylaseand urine catecholamines.Following A&T the authors demonstrate improvement in enuresis within 1month. Although saliva alpha-amylase was reduced after A&T urinecatecholamines remained unchanged.The hypothesis that sleep disordered breathing is related to nocturnal enuresisis not novel. This paper confirms previous findings of resolution of enuresis afterA&T but also offers further evidence supporting a role for the autonomic nervoussystem in these processes. Although the role of saliva alpha-amylase as abiomarker of sympathetic activity is not adequately substantiated its samplingand assaying is easy and thus may constitute an attractive tool in enuresisresearch.