Background
The GOLIATH study is a 2-yr trial comparing transurethral resection of prostate (TURP) to photoselective vaporization with the GreenLight XPS Laser System (GL-XPS) for the treatment of benign prostatic obstruction (BPO). Noninferiority of GL-XPS to TURP was demonstrated based on a 6-mo follow-up from the study.
Objective
To determine whether treatment effects observed at 6 mo between GL-XPS and TURP was maintained at the 2-yr follow-up.
Design, setting, and participants
Prospective randomized controlled trial at 29 centers in nine European countries involving 281 patients with BPO.
Intervention
Photoselective vaporization using the 180-W GreenLight GL-XPS or conventional (monopolar or bipolar) TURP.
Outcome measurements and statistical analysis
The primary outcome was the International Prostate Symptom Score for which a margin of three was used to evaluate the noninferiority of GL-XPS. Secondary outcomes included Qmax, prostate volume, prostate specific antigen, Overactive Bladder Questionnaire Short Form, International Consultation on Incontinence Questionnaire Short Form, occurrence of surgical retreatment, and freedom from complications.
Results and limitations
One hundred and thirty-six patients were treated using GL-XPS and 133 using TURP. Noninferiority of GL-XPS on International Prostate Symptom Score, Qmax, and freedom from complications was demonstrated at 6-mo and was sustained at 2-yr. The proportion of patients complication-free through 24-mo was 83.6% GL-XPS versus 78.9% TURP. Reductions in prostate volume and prostate specific antigen were similar in both arms and sustained over the course of the trial. Compared with the 1st yr of the study, very few adverse events or retreatments were reported in either arm. Treatment differences in the Overactive Bladder Questionnaire Short Form observed at 12-mo were not statistically significant at 24-mo. A limitation was that patients and treating physicians were not blinded to the therapy.
Conclusions
Twenty-four-mo follow-up data demonstrated that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits efficacy and safety outcomes similar to TURP.
Patient summary
The long-term effectiveness and safety of GLP-XLS was similar to conventional TURP for the treatment of prostate enlargement.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
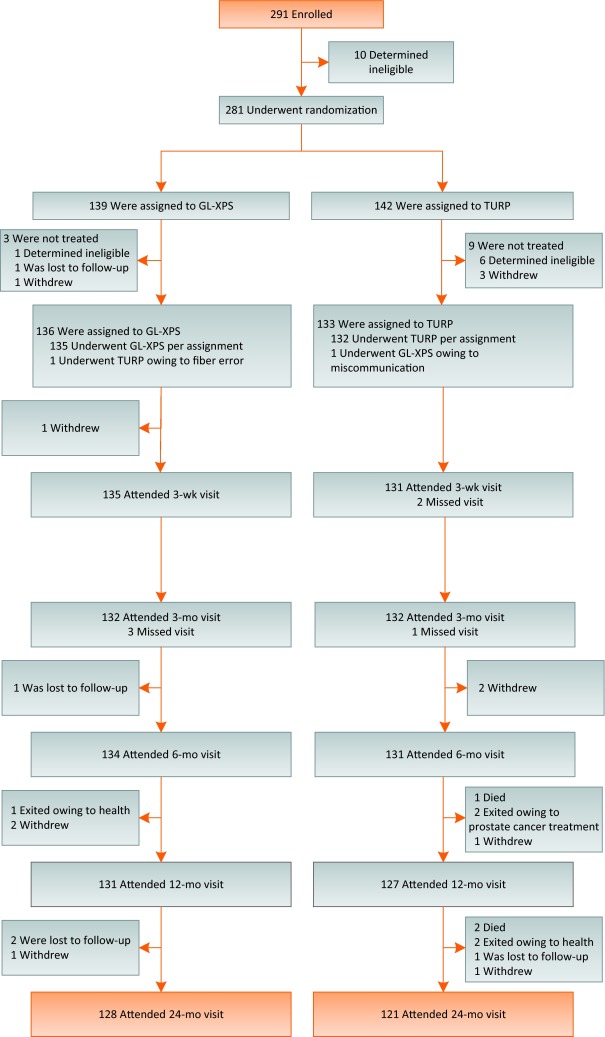
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.
Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
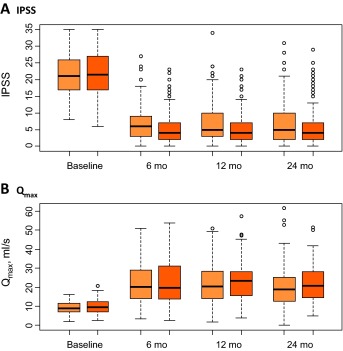
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.
There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).
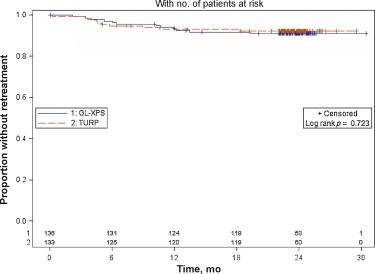
Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.
The incidence of benign prostatic hyperplasia (BPH) is 50–60% in the 6th decade of life and increases to 80–90% in the 7th and 8th decades of life [1]. Due to the progressive nature of the disease, many men initially treated with conservative therapies require surgical intervention to relieve their symptoms. Previously, we reported [2] and [3] the 6-mo and 12-mo results of a clinical trial comparing GreenLight XPS Laser System (GL-XPS) with transurethral resection of prostate (TURP) for efficacy and safety (The GOLIATH study) in the treatment of benign prostatic obstruction (BPO). This large, prospective, randomized study demonstrated that GL-XPS vaporization of the prostate (PVP) was noninferior to TURP with respect to International Prostate Symptom Scores (IPSS), Qmax, and proportion of patients free of complications. Time-to-stable health status, catheterization time, length of hospitalization, and immediate surgical reintervention rates (within 30 d postoperation) were statistically significant in favor of GL-XPS.
Herein we report 2-yr study results with an emphasis on the assessment of durability: sustained reduction in BPO signs and symptoms, retreatments, quality of life (QoL), and safety. This analysis is intended to provide practitioners with contemporary high quality long-term data on the outcomes of both techniques.
To be randomized, patients had to be candidates for the surgical relief of BPO, with IPSS scores of ≥12 and prostate sizes ≤100 g. The complete list of inclusion/exclusion criteria was previously published [2] (see Supplementary data). The trial was conducted under the oversight of country specific ethics committees, and all patients underwent an ethics committee approved informed consent process. The enrollment of patients was the responsibility of the principal investigator at each clinical center. The trial was registered at www.clinicaltrials.gov (NCT01218672).
The study was an open-label, multicenter, prospective, randomized, and controlled noninferiority trial comparing GL-XPS and TURP with a 24-mo follow-up. The trial was conducted at 29 centers in nine European countries with the primary endpoint being IPSS at 6-mo. Secondary outcomes included assessments of BPH Impact Index (assessed up to Mo 3), Qmax, proportion of patients classified as complication-free, post void residual (PVR), prostate-specific antigen (PSA), and prostate volume ascertained with transrectal ultrasonography. Lower urinary tract symptoms, erectile function, and QoL were assessed using validated questionnaires (Overactive Bladder Questionnaire–short form Symptoms [OABq-SF], OABq-SF Health, International Consultation on Incontinence Questionnaire-Urinary Incontinence-Short Form [ICIQ-UI-SF], Short Form-36 Health Survey [SF-36], International Index of Erectile Function-5). All adverse events were adjudicated by an independent clinical events committee (CEC) as described previously [2].
All surgeons were licensed urologists experienced with TURP. Surgeons performing GL-XPS underwent prespecified training on a well published GL technique [4] that was standardized across sites.
Patients were assigned to treatments following a computer generated 1:1 randomization schedule with varying block sizes of two and four, stratified by center. The treatment assignments were prepared centrally by the study sponsor, sealed in opaque, sequentially numbered envelopes, and opened by trained center staff at the time of randomization. The study was designed to provide 80% power to evaluate noninferiority of GL-XPS compared with TURP for each of IPSS, Qmax, and complication-free proportion at 6-mo. The methods used to assess noninferiority were described previously [2], and the primary analysis was performed according to treatment actually received with intention-to-treat as a sensitivity analysis. For all other comparisons, statistical significance was assessed at the 5% level (two-sided) using the two-sample t test or Wilcoxon Rank Sum test for continuous variables and the Fisher exact test for categorical variables. All statistical analyses were performed using SAS 9.2 (Cary, NC, USA).
Two hundred and ninety-one patients were enrolled between April 2011 and September 2012. Two hundred and eighty-one patients were randomly assigned to GL-XPS (n = 139) or TURP (n = 142) (Fig. 1). One hundred and thirty-six patients and 133 patients underwent treatment with GL-XPS or TURP, respectively. At the 2-yr follow-up 93% of patients were available for analysis: 128 GL-XPS patients (94%) and 121 TURP patients (91%). The mean duration of follow-up was similar between groups: 23.3 (standard deviation [SD] 3.1 mo; range, 0.1–30.5 mo) mo and 23.2 (SD 3.6 mo; range, 5.1–29.5 mo) mo for GL-XPS and TURP, respectively.

Baseline characteristics between treatment groups were comparable as previously described [2] (see Supplementary data). Mean IPSS was 21.2 (SD 5.9) in GL-XPS and 21.7 (SD 6.4) in TURP; Qmax (ml/s) was 9.5 (SD 3.0) and 9.9 (SD 3.5).
Results from the study after 6 mo and after the 1st yr were previously reported [2] and [3]. Noninferiority of GL-XPS was demonstrated on each IPSS, Qmax, and proportion of patients classified as complication-free based on the analysis performed by treatment received (Table 1); the intention-to-treat analyses gave the same conclusions. The IPSS and IPSS-QoL values decreased from baseline at similar magnitudes comparing treatment groups (Table 1 and Table 2; Fig. 2).
Table 1 Evaluating noninferiority of GreenLight XPS Laser Systema
| Endpoint | Time point | GL-XPSb | TURPb | Difference [95% CI]c |
|---|---|---|---|---|
| IPSS score | Baseline | 21.2 ± 5.9 | 21.7 ± 6.4 | |
| 6 mo | 6.8 ± 5.2 | 5.6 ± 4.9 | 1.2 [-0.0, 2.4]d | |
| 12 mo | 6.9 ± 6.0 | 5.7 ± 5.3 | 1.2 [-0.2, 2.6]d | |
| 24 mo | 6.9 ± 6.0 | 5.9 ± 6.1 | 1.0 [-0.5, 2.5]d | |
| Qmax(ml/s) | Baseline | 9.5 ± 3.0 | 9.9 ± 3.5 | |
| 6 mo | 23.3 ± 10.1 | 24.3 ± 11.4 | -1.0 [-3.7, 1.8]d | |
| 12 mo | 22.9 ± 10.7 | 24.7 ± 10.1 | -1.7 [-4.5, 1.0]d | |
| 24 mo | 21.6 ± 10.7 | 22.9 ± 9.3 | -1.3 [-4.0, 1.4]d | |
| Complication-free | 6 mo | 87.3% | 83.3% | 4.0% [-4.6%, 12.6%]d |
| 12 mo | 84.7% | 80.5% | 4.3% [-5.0%, 13.6%] | |
| 24 mo | 83.6% | 78.9% | 4.7% [-5.0%, 14.4%]d |
a Intention-to-treat analysis was also performed for IPSS and Qmax using each of two approaches for the patients who did not receive treatment in the study: excluding the patients (ie, modified ITT) and imputing their endpoint with the baseline observation. The analyses by intention-to-treat and by treatment received were in agreement.
b Mean ± SD presented for IPSS and Qmax; proportion presented for complication-free.
c Noninferiority margins (GL-XPS minus TURP): 3 for IPSS, -5.0 ml/s for Qmax, and -5% for complication-free endpoint.
d Noninferiority demonstrated.
CI = confidence interval; GL-XPS = GreenLight XPS Laser System; IPSS = International Prostate Symptom Score; ITT = intention-to-treat; SD = standard deviation; TURP = transurethral resection of prostate.
Table 2 Prostate volume, postvoid residual, prostate specific antigen, and International Prostate Symptom Score-Quality of Life
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| Prostate volume (TRUS; ml) | |||
| Baseline | 48.6 ± 19.2 | 46.2 ± 19.1 | |
| n = 136 (45.4, 34.7–62.4) | n = 133 (42.5, 30.0–57.7) | ||
| 6 mo | 23.0 ± 11.7 | 20.5 ± 11.7 | 0.09 |
| n = 132 (22.6, 13.7–30.1) | n = 127 (17.1, 11.5–28.2) | ||
| 12 mo | 21.9 ± 11.0 | 21.0 ± 12.7 | 0.6 |
| n = 100 (20.1, 13.1–28.9) | n = 102 (17.0, 11.7–27.3) | ||
| 24 mo | 23.9 ± 13.0 | 22.4 ± 13.3 | 0.4 |
| n = 123 (21.7, 13.2–32.8) | n = 117 (18.0, 12.0–29.4) | ||
| PVR (ml) | |||
| Baseline | 110.1 ± 88.5 n = 131 (91.9, 40.0 - 157.0) |
109.8 ± 103.9 n = 128 (75.0, 34.8 - 156.5) |
|
| 6 mo | 38.4 ± 50.0 | 34.6 ± 50.6 | 0.5 |
| n = 132 (23.5, 0.9 - 50.8) | n = 129 (20.0, 0.0 - 44.5) | ||
| 12 mo | 42.8 ± 56.9 | 33.4 ± 43.7 | 0.09 |
| n = 129 (25.0, 5.1 - 51.3) | n = 125 (15.9, 0.0 - 52.0) | ||
| 24 mo | 45.6 ± 65.5 | 34.9 ± 47.1 | 0.2 |
| (128 (25.0, 3.3 - 50.9) | n = 119 (20.0, 0.0 - 50.0) | ||
| PSA (ng/ml) | |||
| Baseline | 2.7 ± 2.1 | 2.6 ± 2.1 | |
| n = 136 (2.1, 1.1–3.8) | n = 133 (2.2, 0.9–3.9) | ||
| 6 mo | 1.4 ± 1.5 | 1.0 ± 0.9 | 0.006 |
| n = 130 (0.9, 0.5–1.7) | n = 127 (0.7, 0.4–1.1) | ||
| 12 mo | 1.3 ± 1.3 | 1.1 ± 1.0 | 0.2 |
| n = 129 (0.9, 0.5–1.6) | n = 126 (0.8, 0.5–1.4) | ||
| 24 mo | 1.4 ± 1.7 | 1.1 ± 0.9 | 0.09 |
| n = 126 (0.8, 0.5–1.5) | n = 119 (0.8, 0.5–1.4) | ||
| IPSS-QoL | |||
| Baseline | 4.6 ± 1.1 | 4.5 ± 1.4 | |
| n = 134 (5.0, 4.0–5.0) | n = 132 (5.0, 4.0–6.0) | ||
| 6 mo | 1.5 ± 1.4 | 1.2 ± 1.2 | 0.1 |
| n = 134 (1.0, 0.0–2.0) | n = 130 (1.0, 0.0–2.0) | ||
| 12 mo | 1.4 ± 1.4 | 1.2 ± 1.3 | 0.2 |
| n = 129 (1.0, 0.0–2.0) | n = 126 (1.0, 0.0–2.0) | ||
| 24 mo | 1.3 ± 1.2 | 1.2 ± 1.3 | 0.6 |
| n = 127 (1.0, 0.0–2.0) | n = 120 (1.0, 0.0–2.0) | ||
a Results are represented as mean ± standard deviaion, n (median, first quartile–third quartile).
GL-XPS = GreenLight XPS Laser System; IPSS-QoL = International Prostate Symptom Score-Quality of Life; PSA = prostate-specific antigen; PRV = postvoid residual; TRUS = transrectal ultrasound; TURP = transurethral resection of prostate.

There were a few differences in other secondary variables including similar reductions in prostate size and PVR relative to baseline in both treatment arms comparing 6-mo and 12-mo results (Table 2). Mean PSA values were reduced by approximately 50% posttreatment for both groups, and PSA was not statistically different between treatments at any time point except for mo-6 (p = 0.006) (Table 2). At mo-3, mean Benign Prostatic Hyperplasia Impact Index scores were 2.6 (SD 2.9) in GL-XPS and 2.2 (SD 2.9) in TURP compared with 7.1 (SD 3.1) in GL-XPS and 6.8 (SD 3.1) in TURP at baseline. As described previously [2] and [3], time-to-stable health status, catheterization time, and length of hospitalization, were statistically significant in favor of GL-XPS (p < 0.001),. Immediate surgical reintervention rates (within 30 d postoperation) were higher in TURP however the overall reintervention rates were not significantly different between treatment arms.
The mean IPSS at 24-mo remained at the lower, postoperative level (6.9 GL-XPS vs 5.9 TURP; difference between arms: 1.0, 95% confidence interval [CI]: -0.5 to +2.5); noninferiority of GL-XPS was demonstrated (Table 1; Fig. 2). Similarly, the mean Qmax remained at clinically higher levels in both treatment groups (21.6 GL-XPS vs 22.9 TURP; difference: -1.3, 95% CI: -4.0 to 1.4); noninferiority of GL-XPS was maintained at 24-mo (Table 1). Noninferiority was also maintained for the proportion of patients classified as complication-free in the GL-XPS group (83.6%) compared with the TURP group (78.9%); difference: 4.7%, 95% CI:-5.0% to 14.4% (Table 1).
After 2 yr, mean PSA values remained approximately 50% lower compared with baseline and the groups were not significantly different (Table 2). Similar reductions in prostate size and PVR were observed in the two groups and the groups were not statistically significantly different at the 2-yr visit. Results of QoL assessments showed no statistically significant differences between treatment groups after 24-mo. This included OABq-SF symptoms, OABq-SF health, ICIQ-UI-SF (Table 3), as well as EQ-5D, SF-36 Mental Health, and SF-36 Physical Health (see Supplementary data). Sexual satisfaction was not statistically different between treatments based on International Index of Erectile Function-5 (Table 3). Patient satisfaction with their treatment measured by: (1) willingness to undergo the therapy again was 93% in GL-PVP and 89% in TURP; and (2) willingness to recommend their therapy was similar (93% in GL-PVP and 91% in TURP) between techniques at 2-yr.
Table 3 Over active bladder, urinary incontinence questionnaires, and erectile function
| Scale/time point | GL-XPSa | TURPa | p value |
|---|---|---|---|
| OABq-SF symptoms | |||
| Baseline | 44.2 + 20.5 (134) (43.3, 30.0–56.7) |
42.9 + 20.8 (132) (43.3, 26.7–60.0) |
|
| 6 mo | 16.6 ± 16.2 | 11.5 ± 13.1 | 0.005 |
| (132) (13.3, 6.7–23.3) | (129) (6.7, 0.0–16.7) | ||
| 12 mo | 16.7 + 18.0 | 12.7 + 14.2 | 0.051 |
| (131) (10.0, 3.3–23.3) | (125) (8.0, 0.0–20.0) | ||
| 24 mo | 15.3 + 16.7 | 11.9 + 13.7 | 0.09 |
| (126) (10.0, 0.0–23.3) | (120) (9.0, 0.0–16.7) | ||
| OABq-SF health | |||
| Baseline | 59.0 ± 21.9 | 62.6 ± 21.7 | |
| (136) (61.5, 44.8–75.4) | (131) (64.6, 51.7–78.5) | ||
| 6 mo | 87.3 + 15.9 | 90.9 + 13.4 | 0.049 |
| (133) (93.8, 81.7–98.5) | (129) (96.7, 89.1–100.0) | ||
| 12 mo | 87.1 + 17.7 | 91.4 + 12.8 | 0.03 |
| (131) (93.8, 83.1–100.0) | (122) (96.7, 89.2–100.0) | ||
| 24 mo | 88.5 + 15.8 | 91.1 + 13.7 | 0.2 |
| (127) (95.4, 83.1–98.5) | (120) (95.4, 89.2–100.0) | ||
| ICIQ-UI SF | |||
| Baseline | 3.9 ± 4.7 | 4.4 ± 4.6 | |
| (131) (3.0, 0.0–7.0) | (128) (4.0, 0.0–7.0) | ||
| 6 mo | 3.0 + 4.1 | 1.7 + 2.8 | 0.004 |
| (132) (0.0, 0.0–5.0) | (128) (0.0, 0.0–3.0) | ||
| 12 mo | 3.3 + 4.5 | 2.1 + 3.3 | 0.02 |
| (128) (0.0, 0.0–5.0) | (122) (0.0, 0.0–4.0) | ||
| 24 mo | 2.8 + 4.1 | 2.0 + 3.3 | 0.1 |
| (122) (0.0, 0. 0.0–4.0) | (118) (0.0, 0.0–4.0) | ||
| IIEF-5 | |||
| Baseline | 13.2 ± 7.6 | 13.7 ± 7.5 | |
| (132) (14.0, 6.5–20.0) | (129) (15.0, 7.0–19.0) | ||
| 12 mo | 12.9 + 7.5 | 14.2 + 8.2 | 0.2 |
| (129) (14.0, 6.0–19.0) | (121) (17.0, 5.0–21.0) | ||
| 24 mo | 12.9 + 7.5 | 13.9 + 8.2 | 0.3 |
| (124) (13.5, 6.0–19.0) | (119) (15.0, 6.0–21.0) | ||
a Results are represented as mean ± standard deviation (n) (median, first quartile–third quartile).
ICIQ-UI SF = International Consultation on Incontinence Questionnaire-Urinary Incontinence short form; IIEF-5 = International Index of Erectile Function-5; GL-XPS = GreenLight XPS Laser System; OABq-SF = Overactive Bladder Questionnaire–short form; TURP = transurethral resection of prostate.
All adverse events (AEs) classified as treatment-related by the CEC during the 0-mo to 12-mo period were previously described [2] and [3]. AEs were classified using the Clavien-Dindo [5] scale (Grade I–V). During the 2nd yr of the study, there were few AE reported incidents (Table 4). Five GL-XPS patients accounted for five events: one Grade I irritative symptom, two Grade II urinary tract infections, one Grade IIIa stricture (bladder neck), and one Grade IIIb urinary retention incident. In the TURP group, two patients accounted for two events: one Grade I other (worsening erectile function) and one Grade IIIa urinary retention incident.
Table 4 Adverse events in each study period
| Mo 0–6 | Mo 7–12 | Mo 13–24 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts a | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Clavien-Dindo Grade I | ||||||||||||
| Bleeding | 9 | 9 | 10 | 10 | ||||||||
| Urinary tract infection | 6 | 5 | 2 | 2 | ||||||||
| Irritative symptoms b | 24 | 21 | 24 | 24 | 3 | 3 | 1 | 1 | ||||
| Stricture c | 1 | 1 | ||||||||||
| Urinary incontinence | 10 | 10 | 4 | 4 | 2 | 2 | ||||||
| Urinary retention | 8 | 8 | 3 | 3 | ||||||||
| Other | 6 | 6 | 5 | 5 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade II | ||||||||||||
| Bleeding | 3 | 3 | 2 | 2 | ||||||||
| Urinary tract infection | 19 | 19 | 14 | 12 | 2 | 2 | 2 | 2 | ||||
| Irritative symptoms b | 6 | 6 | 5 | 5 | ||||||||
| Urinary incontinence | 5 | 4 | 2 | 2 | ||||||||
| Other | 2 | 2 | 2 | 2 | ||||||||
| Clavien-Dindo Grade IIIa | ||||||||||||
| Bleeding | 1 | 1 | 4 | 4 | 1 | 1 | ||||||
| Irritative symptoms b | 1 | 1 | ||||||||||
| Stricture c | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Urinary retention | 6 | 6 | 10 | 9 | 1 | 1 | 1 | 1 | ||||
| Clavien-Dindo Grade IIIb | ||||||||||||
| Bleeding | 2 | 2 | 6 | 6 | ||||||||
| Stricture c | 4 | 4 | 5 | 5 | 3 | 3 | 2 | 2 | ||||
| Urinary retention | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Total | 112 | 69 | 100 | 64 | 14 | 12 | 5 | 5 | 5 | 5 | 2 | 2 |
a The total number of patients with at least one event in the grade will not equal the sum of number of patients in each category when a patient has an event in more than one category within the grade.
b Irritative symptoms include pain and discomfort.
c Includes meatal, urethral, and bladder neck stricture.
# = number of event; GL-XPS = GreenLight XPS Laser System; TURP = transurethral resection of prostate.
During Mo 0–12, there were 19 retreatment surgeries: 10 for GL-XPS patients and nine for TURP patients. During the 13–24 mo period, there were five additional cases: four for GL-XPS patients and one for a TURP patient involving prostate tissue regrowth, bladder neck contracture, or urethral stricture (Fig. 3; see supplementary data). The Kaplan Meier estimates for reoperation by 24-mo are 9.0% for GL-XPS and 7.6% for TURP (Fig. 3); these are not statistically different (p = 0.7, log rank test) (Table 5).

Table 5 Surgical retreatments for obstructiona
| Study period | Mo 0–6 | Mo 7–12 | Mo 13–24 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GL-XPS | TURP | GL-XPS | TURP | GL-XPS | TURP | |||||||
| Event | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts | # | Pts |
| Prostate tissue regrowth | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||
| Bladder neck contracture | 2 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 3 | 3 | ||
| Urethral stricture | 1 | 1 | 4 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Other | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| Total surgeries | 4 | 4 | 7 | 7 | 6 | 6 | 2 | 2 | 4 | 4 | 1 | 1 |
a Surgical retreatments: events treated to alleviate voiding obstruction.
# = number of events; GL-XPS = GreenLight XPS Laser System; Pts = patients; TURP = transurethral resection of prostate.
Urinary incontinence (UI) was based on self-reporting of any drop of urine leakage postsurgical treatment. The prevalence and grade of UI at 24-mo were unchanged from those observed at 12-mo (four patients in each of GL-XPS and TURP).
If prostate cancer was identified during a procedure or if a physician became aware of a diagnosis during any follow-up visit then the prostate cancer was reported as an AE. In the TURP arm, prostate cancer was reported in five patients at the time of procedure, one within the first 12-mo and one at 18-mo. There were no reports of prostate cancer in the GL-XPS arm.
In recent yr there has been an ever-increasing use of lasers in medicine particularly in oncology, cardiovascular disease, ophthalmology, and urology. Laser prostatectomy has become a prominent surgical therapy for BPO and is included in most BPO guidelines [6]. The present study suggests that the outcomes for GL-XPS are similar to TURP with shorter hospital stays and length of catheterization. Previously published evaluations of laser systems have been less than adequate due to several methodological flaws in study design including high drop-out rates during follow-up, short duration of follow-up, and/or being mainly single-center–single surgeons experiences [6] and [7]. In contrast, the current study was adequately powered to investigate its primary outcome measures and benefits from being conducted in 29 centers across nine European countries. The GOLIATH-Study was designed to represent local preferences within the large range of European countries. Moreover, a very high proportion of patients in the cohort (92%) remained in the study until the 24-mo visit allowing for a thorough assessment of durability and safety. Noninferiority of GL-XPS was demonstrated for each of IPSS, Qmax, and complication-free rate at 6-mo follow-up and was maintained at 24-mo.
Although GOLIATH is the largest randomized controlled trial to compare laser prostatectomy with TURP for efficacy and safety, some have expressed concern over the duration of follow-up and whether it is adequate to demonstrate durability of response. Long-term efficacy and safety and the need for surgical retreatment are central to the discussion of overall relative value of minimally invasive procedures compared to TURP. While the most informative studies have a follow-up of 5 yr or more, there is evidence that under certain circumstances 2-yr follow-up data is representative of what will be observed at 5-yr of follow-up [8]. Specifically, if the surgical reoperation rate is both low and stable and the immediate improvement in efficacy is maintained at 2-yr, then it is very likely that a 5-yr follow-up will be consistent with a durable response [9].
In the current study, the 2-yr Kaplan-Meier estimates for surgical reoperation were 9.0% for GL-XPS and 7.6% for TURP, which are similar and not statistically different. It is of note that these rates are low compared with current literature and stable after 12-mo postprocedure. The improvements observed in IPSS, IPSS-QoL, and Qmax at 6-mo are maintained for the duration of the follow-up. The comparison of the functional outcomes appears to slightly favor patients treated with TURP but treatment differences were not statistically significant or clinically meaningful. Furthermore, based on previous reports in literature, there is every reason to anticipate that the beneficial clinical outcomes observed at 2 yr will also be prevalent 5 yr after surgery. Taken together, these observations suggest that GL-XPS is a durable, effective, and safe therapy for surgical management of BPH.
Previous reports have suggested that the amount of tissue removed during GL-XPS is significantly less than that removed during a TURP and can result in a higher rate of treatment failure. Objective measurements of prostate volume change were not statistically different comparing the two arms of the study at 24-mo. Moreover, serum PSA decline and nadir is not statistically different between the two groups.
A noteworthy observation is the time-course of change in irritative symptoms. UI and overactive bladder were assessed using validated questionnaires at baseline, 6-mo, 12-mo, and the end of study. At 6 mo postoperatively overactive blader-related symptom scores (OABq-SF Symptom/OABq-SF Health) and incontinence-related symptom scores (ICIQ-UI SF) were significantly better; however, not clinically meaningful in the TURP group compared with the GL-XPS arm [10] and [11]. By 24-mo there was no statistical difference between the two groups suggesting that any observed differences in irritative symptoms are of limited clinical significance (Table 3).
Due to technological differences between the treatments, blinding was not attempted. To minimize possible bias, the study had a detailed protocol, with common training provided to all investigators. An independent CEC adjudicated all AEs in blinded fashion.
To date, this study is the largest prospective randomized trial comparing TURP with laser prostatectomy, providing data on outcomes after surgical treatment of BPO. Two-yr follow-up data demonstrate that GL-XPS provides a durable surgical option for the treatment of BPO that exhibits similar efficacy and safety outcomes to TURP.
Author contributions: James A. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Bachmann.
Acquisition of data: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Analysis and interpretation of data: Thomas, Bachmann.
Drafting of the manuscript: Thomas, Bachmann.
Critical revision of the manuscript for important intellectual content: Thomas, Tubaro, Barber, d’Ancona, Muir, Witzsch, Grimm, Benejam, Stolzenburg, Riddick, Pahernik, Roelink, Ameye, Saussine, Bruyère, Loidl, Larner, Gogoi, Hindley, Muschter, Thorpe, Shrotri, Graham, Hamann, Miller, Schostak, Capitán, Knispel, Bachmann.
Statistical analysis: Thomas, Bachmann, American Medical Systems-Statistician.
Obtaining funding: American Medical Systems.
Administrative, technical, or material support: Thomas, Bachmann, American Medical Systems.
Supervision: Thomas, Bachmann.
Other (Summer Creek Consulting, LLC): Stein.
Financial disclosures: James A. Thomas certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: James A. Thomas and Alexander Bachmann and are the European Joint principal investigators of the Goliath study; both are advisers for AMS and received honoraria for presentations. The other authors have nothing to disclose.
Funding/Support and role of the sponsor: American Medical Systems (AMS) helped designed and conduct the study, collect, manage, and analyze the data, and prepare, review, and approve the manuscript. This study was sponsored by an AMS clinical grant (NCT01218672).
Acknowledgments: The GOLIATH study team thanks all the patients in nine European countries who were willing to participate in this unique study. The joint principal investigators (PIs) of the GOLIATH study are pleased to thank all the center PIs, surgeons, attending colleagues, study nurses, nurses, study monitors, and supporters of GOLIATH. Special thanks to Prof. C. Chapple, Prof. M. Marberger, and Prof. O. Reich for their function as the independent Adverse Event Adjunction Committee. Both joint PIs thank especially the AMS GOLIATH team for their excellent and consistent administrative and statistical support and finally the study management.