α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.
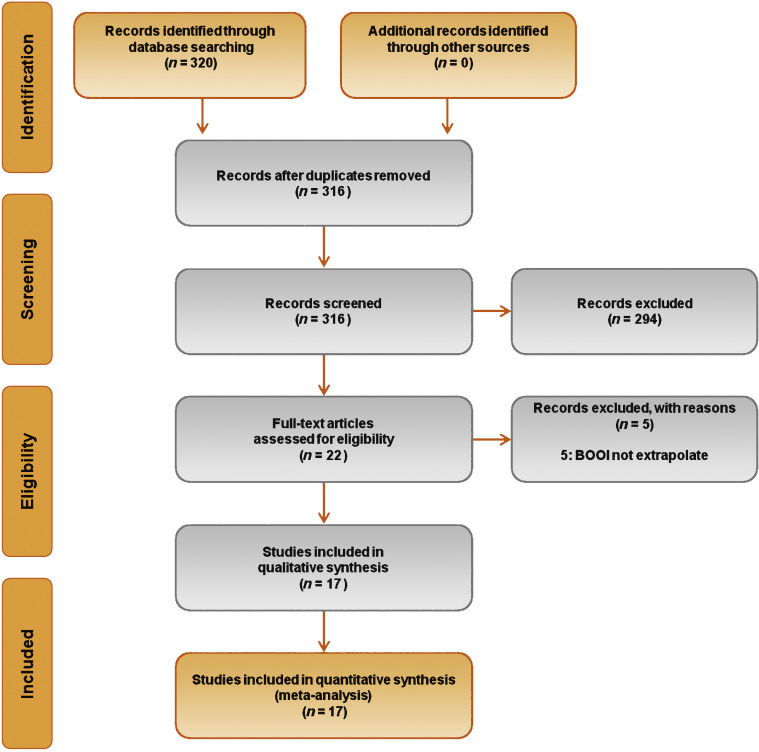
Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
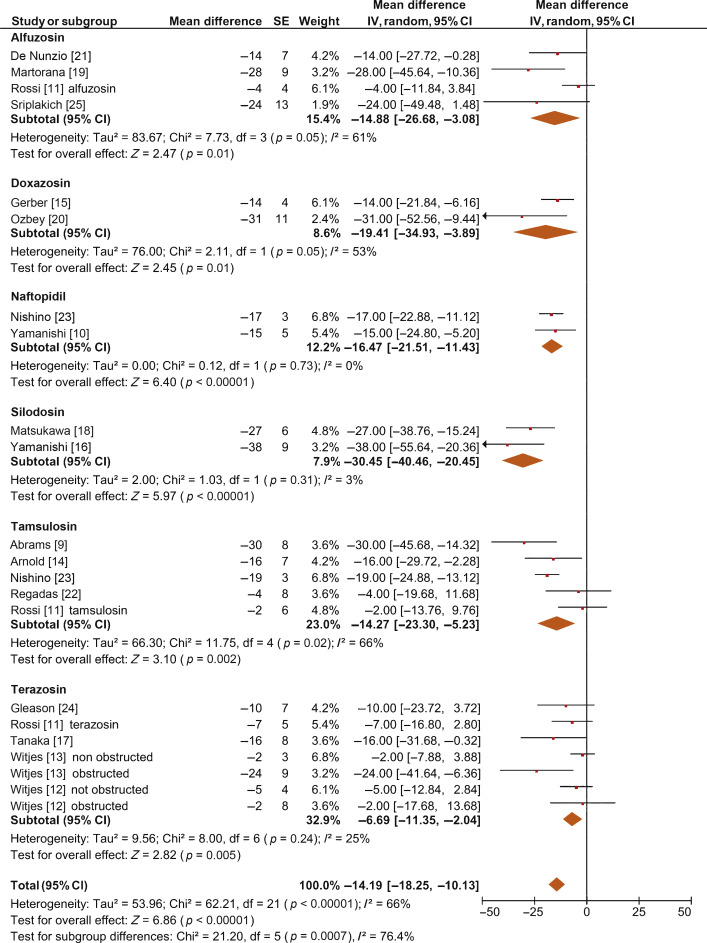
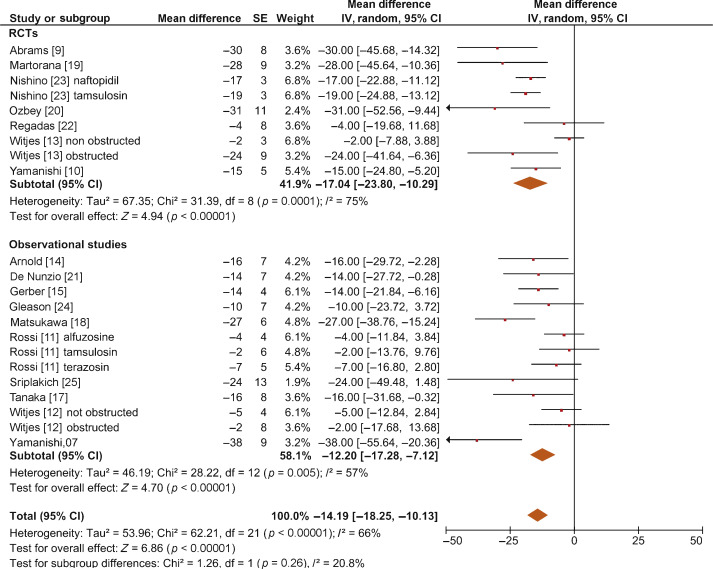
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).
Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).
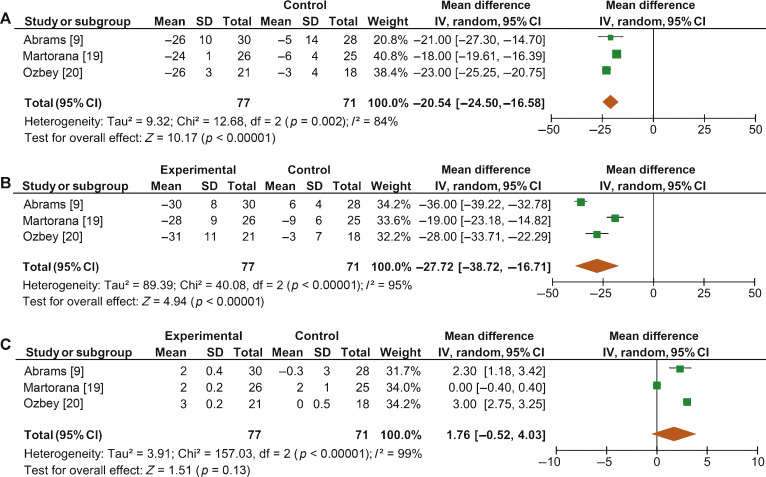
Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
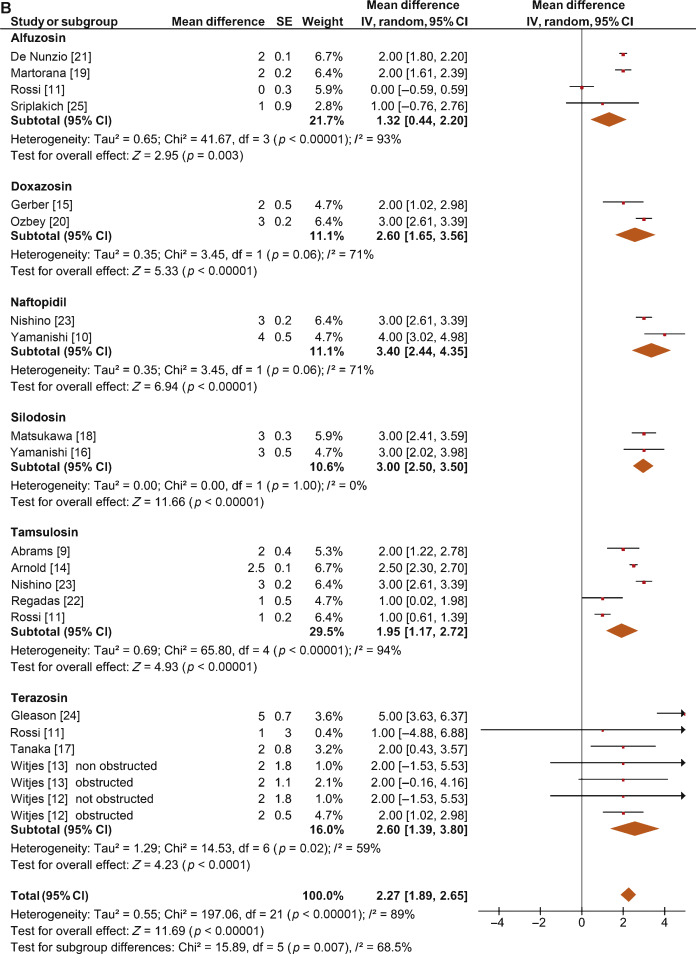
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).
Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.
α1-Blockers (ABs) are frequently prescribed as first-line therapy for the treatment of moderate to severe lower urinary tract symptoms related to benign prostatic enlargement (LUTS/BPE) [1] and [2]. To date, six ABs have been approved for the treatment of LUTS/BPE: terazosin, doxazosin, tamsulosin, naftopidil, alfuzosin, and silodosin. All of them have been reported to significantly improve voiding and storage LUTS with respect to placebo [2]. Historically, it has been assumed that the pathophysiology of LUTS/BPE is the result of benign prostatic obstruction (BPO). Consequently, it was generally presumed that LUTS/BPE improvements on ABs treatment were due to a reduction in BPO mediated by relaxation of prostatic smooth muscle. In recent years, various lines of evidence have questioned this paradigm. Studies have underlined that symptom score, peak urinary flow rate at free uroflowmetry (free Qmax), and BPO represent different aspects of LUTS that are only poorly related to each other [3]. Published data support the common belief that ABs have a minimal effect on urinary flow rate and therefore a minimal impact on BPO [2]. It has also been hypothesized that the mechanisms underlying the beneficial effects of ABs may be more complex than previously assumed, and that α1-adrenoceptors located outside the prostate (eg, urinary bladder and/or spinal cord) may play a role [4]. However, BPO remains a key issue when dealing with patients with BPE. A correct diagnosis of BPO requires an invasive pressure/flow study (PFS) in which urodynamic Qmax and detrusor pressure at Qmax (PdetQmax) are measured and used to calculate the bladder outlet obstruction index (BOOI). Obstruction is defined as a high-pressure/low-flow micturitional pattern and is diagnosed when the BOOI is >40. Although the BOOI is recommended for measuring the level of obstruction, most studies evaluating therapy with ABs for LUTS/BPE confined analyses to free uroflowmetry, symptom score, and postvoid residual urine (PVR) [5] and [6]. Conversely, only a few high-quality studies have evaluated the urodynamic outcomes of AB treatment for PFS parameters in patients suffering from LUTS/BPE, and the results have been inconclusive [3] and [5]. We performed a meta-analysis of published studies to clarify the urodynamic outcomes of ABs treatment on BOOI and other major PFS urodynamic parameters in patients with LUTS/BPE.
This analysis was conducted and reported according to the general guidelines recommended by the Primary Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [7].
In May 2015 we used the National Library of Medicine PubMed search engine, the Scopus database, and the ISI Web of Knowledge official website to search for all published studies evaluating urodynamic measurement of BOOI in LUTS/BPE patients before and after AB therapy. The followings search strings were used: tamsulosin AND urodynamics; silodosin AND urodynamics; alfuzosin AND urodynamics; doxazosin AND urodynamics; naftopidil AND urodynamics; and terazosin AND urodynamics. We included publications that met the following criteria: reporting original research; English language; human studies; enrolling LUTS/BPE patients; and reporting Qmax and PdetQmax evaluated by PFS before and after treatment with an AB. Reference lists in relevant articles and reviews were also screened for additional studies. Abstracts (with no subsequent full-text publications) and unpublished studies were not considered. Two authors (F.F., M.C.) reviewed the records separately to select relevant publications, with any discrepancies resolved by open discussion. The quality of the randomized controlled trials (RCTs) was assessed using the Jadad score [8].
The following data were extracted from the studies included: publication year; study design; sample size; number of patients with obstruction at baseline; type of AB used; duration of treatment; and PdetQmax and Qmax values at baseline and after treatment. PdetQmax and Qmax values at baseline and after treatment were also extracted from the placebo arms when available. BOOI was calculated using the formula BOOI = PdetQmax – 2Qmax[6]. The number and percentage of patients with obstruction at baseline who changed their class of obstruction from “obstructed” to “non-obstructed” or “equivocal” was also extracted.
The primary outcome was change in BOOI. Changes in PdetQmax and Qmax were evaluated as secondary outcomes.
Continuous variables are reported as mean difference (MD) estimate, standard error, inverse-variance weight, and 95% confidence intervals (CIs) for each study. Statistical pooling for MD estimates was performed according to a random-effects model with generic inverse-variance weighting, computing estimates with 95% CI, using Review Manager Software 5 (The Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Study bias was appraised by graphical inspection of funnel plots. Hypothesis testing for superiority was set at a two-tailed level of 0.05. Hypothesis testing for statistical homogeneity was set at a two-tailed level of 0.10 and was based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and extensive statistical inconsistency, respectively. Forest plots were generated to show changes in BOOI, Qmax, and PdetQmax during the assumption of AB versus baseline. For the placebo-controlled RCTs, a forest plot was also generated showing the change in BOOI during the assumption of AB versus placebo. A meta-regression analysis was used to assess the possible effect of the number of patients with obstruction at baseline on BOOI improvement. Meta-regression analysis was performed using Comprehensive Meta-Analysis, reporting results as β with 95% CI and the significance level observed. Subgroup analyses were performed according to the type of AB. Sensitivity analyses were performed according to the type of study (RCTs and non-RCTs). A pooled analysis of placebo-controlled RCTs was performed to compare ABs with placebo.
Database searches revealed 320 publications up to January 2015. Of these, 298 were immediately excluded according to the title or abstract, or because of duplicated series. A further five papers were excluded because BOOI could not be extrapolated. Finally, 17 studies involving a total of 656 patients were included in the meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], and [25] (Fig. 1). Specifically, seven studies were RCTs and 10 were not randomized prospective studies. Only three of seven RCTs were placebo-controlled. According to the Jadad score, three of the seven RCTs were high-quality studies. The characteristics of the studies included are summarized in Table 1. A funnel plot of all the studies included suggested that publication bias was not present.

Table 1 Characteristics of the studies included in the review
| Study | Design | JS | Sample size ( n) a |
Control group ( n) |
Treatment protocol (drug and dosage) | Treatment (wk) | Obstruction at baseline, n (%) | Obstruction resolved, n (%) b |
|---|---|---|---|---|---|---|---|---|
| Gleason 1994 [24] | NRNCT | NA | 17 | NA | Terazosin 1 mg OAD, titrated up to 2, 5, and 10 mg OAD as tolerated | 8 | NR | NA |
| Witjes 1996 [13] | RCT | 2 | 33 | NA | Terazosin at bedtime and increased to maximum dose 10 mg OAD at 6 wk | 26 | 22 (66.6) | NA |
| Witjes 1997 [12] | NRNCT | NA | 60 | NA | Terazosin increased to maximum of 10 mg OAD at 6 wk | 112 | 30 (50) | NA |
| Tanaka 2002 [17] | NRNCT | NA | 20 | NA | Terazosin 1 mg OAD for the first 7 d and then 1 mg TAD | 4 | 10 (50) | 6 (60) |
| Gerber 1996 [15] | NRNCT | NA | 44 | NA | Doxazosin 1 mg OAD for 4 d, then 2 mg OAD for 4 d, then 4 mg OAD | 12 | 30 (68.1) | 9 (30) |
| Ozbey 1999 [20] | PC-RCT | 2 | 21 | 18 | Doxazosin 2 mg OAD, then 4 mg OAD | 4 | NR | NA |
| Abrams 1997 [9] | DB-PC-RCT | 3 | 30 | 28 | Tamsulosin 0.4 mg OAD | 4 | 30 (100) | NA |
| Arnold 2001 [14] | NRNCT | NA | 28 | NA | Tamsulosin 0.4 mg OAD | 12 | 30 (100) | 21 |
| Regadas 2013 [22] | DB-PC-RCT | 3 | 20 | 20 | Tamsulosin 0.4 mg OAD | 4 | 12 (60) | NA |
| Yamanishi 2004 [10] | SB- RCT | 2 | 24 | 12 | Naftopidil 50–75 mg OAD | 4–6 | 22 (61.1) | 13 (59) |
| Martorana 1997 [19] | DB-PC-RCT | 3 | 25 | 26 | Alfuzosin 2.5 mg TID | 4 | NR | NA |
| Nishino 2006 [23] | CO-RCT | 2 | 34 | NA | Naftopidil 50 mg for 4 wk, followed by tamsulosin 0.2 mg for 4 wk (n = 17) Tamsulosin 0.2 mg for 4 wk, followed by naftopidil 50 mg for 4 wk (n = 17) |
9 | Naftopidil 28 (82.3) Tamsulosin 30 (88.2) |
Naftopidil 21 (75) Tamsulosin 13 (43.3) |
| De Nunzio 2003 [21] | NRCT | NA | 20 | 20 | Alfuzosin SR 5 mg TAD | 96 | 20 (100) | 4, (20) |
| Sriplakich 2007 [25] | NRCT | NA | 13 | 12 | Alfuzosin SR 10 mg OAD | 12 | 25 (100) | NA |
| Rossi 2001 [11] | NRCT | NA | 163 | NA | Alfuzosin 2.5 mg TID (n = 60) Terazosin 5 mg OAD (n = 66) Tamsulosin 0.4 mg. OAD (n = 37) |
24 | NR | NA |
| Matsukawa 2009 [18] | NRNCT | NA | 57 | NA | Silodosin 4 mg TAD | 4 | NR | NA |
| Yamanishi 2010 [16] | NRNCT | NA | 27 | NA | Silodosin 4 mg TAD | 12 | 30 (83) | NA |
a Sample size based on data extracted for meta-analysis may differ from number reported in the original study.
b Percentage of patients who went from the obstructed to the equivocal or unobstructed class.
SR = slow release; OAD = once a day; TAD = twice a day; TID = three times a day; NR = not reported; JS = Jadad score; NA = not applicable; NRNCT = not randomized, not controlled clinical trial; NRCT = not randomized controlled clinical trial; RCT = randomized controlled clinical trial; CO-RCT = crossover RCT; PC-RCT = randomized placebo-controlled clinical trial; DB-PC-RCT = double-blind RP-RCT; SB-RCT = single-blind RCT.
The overall pooled data showed a mean change in BOOI of –14.19 (95% CI –18.25 to –10.13; p < 0.0001; Fig. 2). Subgroup analysis by AB type showed a reduction in BOOI for all ABs. The mean BOOI change observed was –14.88 (95% CI –26.68 to –3.08; p = 0.01) for alfuzosin, –19.41 (95% CI –34.93 to –3.89; p = 0.01) for doxazosin, –16.47 (95% CI –21.51 to –11.43; p < 0.0001) for naftopidil, –30.45 (95% CI –40.46 to –20.45; p < 0.0001) for silodosin, –14.27 (95% CI –23.30 to –5.23; p = 0.002) for tamsulosin, and –6.69 (95% CI –11.35 to –2.04; p = 0.005) for terazosin. Sensitivity analysis by study design showed a mean change in BOOI of –17.04 (95% CI –23.80 to –10.29; p < 0.0001) for RCTs and –12.20 (95% CI –17.28 to –7.12; p < 0.0001) for non-RCTs (Fig. 3).


Meta-regression demonstrated a significant positive association between the percentage of patients with obstruction at baseline and the improvement in BOOI after AB treatment (β –0.17, 95% CI –0.24 to 0.10; p < 0.001).
When we pooled the results for the three RCTs containing a placebo arm, we found a significant improvement in BOOI in patients undergoing treatment with ABs compared to those taking placebo (MD –20.54, 95% CI –24.50 to –16.58; p < 0.0001; Fig. 4A).

Fig. 4 Forest plots for change in (A) Bladder Outlet Obstruction Index, (B) detrusor pressure at maximum urinary flow and (C) maximum urinary flow rate, in the active treatment arm versus placebo arm in placebo-controlled randomized clinical trials. CI = confidence interval; IV = inverse variance; SD = standard deviation.
The overall pooled data showed a mean change in PdetQmax of –11.39 cm H2O (95% CI –15.37 to –7.40; p < 0.0001; Fig. 5A) and a mean improvement in Qmax of 2.27 ml/s (95% CI 1.89–2.65; p < 0.0001; Fig. 5B).

Sensitivity analysis by study design showed a mean change in PdetQmax of –14.46 cm H2O (95% CI –19.85 to –9.06; p < 0.0001) in RCTs and –9.39 cm H2O (95% CI –14.51 to –4.28; p < 0.0001) in non-RCTs. Pooled data from the RCTs containing a placebo arm showed a significant reduction in PdetQmax in patients undergoing treatment with ABs compared to those taking placebo (MD –27.72, 95% CI –38.72 to –16.71; p < 0.0001; Fig. 4B).
Sensitivity analysis by study design showed a mean improvement in Qmax of 2.57 ml/s (95% CI 2.08–3.05; p < 0.0001) in RCTs and 2.07 ml/s (95% CI 1.54–2.60; p < 0.0001) in non-RCTs. Pooled data from RCTs containing a placebo arm failed to show significant differences in Qmax variations among patients undergoing treatment with ABs and placebo (MD 1.76, 95%CI –0.52 to +4.03; p = 0.13; Fig. 4C).
This meta-analysis demonstrates for the first time that ABs can generate significant urodynamic outcomes in patients treated for LUTS/BPE. Interestingly, the meta-analysis showed a statistically significant benefit in favor of AB drugs in terms of BOOI and PdetQmax. This evidence should be strongly considered when counseling LUTS/BPE patients and when the mechanism of action of ABs is discussed, including next guidelines on the management of LUTS/BPE.
ABs are recommended for men with moderate to severe LUTS/BPE and can be prescribed in combination with 5α-reductase inhibitors for men with troublesome moderate to severe LUTS, enlarged prostate, and reduced Qmax[2]. All agents of this class are efficacious in improving both storage and voiding LUTS [2].
Historically, it has been assumed that ABs improve LUTS/BPE by reducing BPO via a relaxing effect on prostatic smooth muscle. In recent years, this paradigm has been questioned. In 2003, Kortmann et al [5] surveyed the published studies available and evaluated all clinical trials in which ABs were tested using urodynamic studies, including PFS. Fifteen studies were included: nine were RCTs, five were open-label studies, and one was of an unspecified design [5]. The authors concluded that ABs led to a small reduction in BPO and a decrease in urodynamic parameters of up to 50% [5]. In 2008, Barendrecht et al [3] performed a retrospective analysis of a previously reported randomized, placebo-controlled, double-blind study that compared urodynamic outcomes between modified-release tamsulosin and placebo. The authors found that LUTS, free Qmax, and BOOI were only loosely related at baseline. More importantly, treatment-induced improvements in these parameters were also only loosely related [3]. The authors therefore questioned the hypothesis that ABs improve LUTS by reducing BPO and suggested that they may also act independently by affecting prostatic smooth muscle tone [3]. However, these two studies had many methodological weaknesses that affect the reliability of the conclusions. The study by Kortmann et al [5] was not a meta-analysis, as the studies were combined in a simple narrative fashion. The ABs evaluated were prazosin, doxazosin, terazosin, alfuzosin, and tamsulosin. These studies reported Qmax and/or PdetQmax and/or maximal voiding detrusor pressure as urodynamic measures. Data were extracted for patients receiving ABs only. The review methodology was not described and appropriate measures to avoid the introduction of errors and bias do not appear to have been taken. The quality of the studies was not assessed, so the reliability of the findings of the studies reviewed was unclear. Finally, despite including placebo-controlled trials, only data from the intervention groups were considered. Conversely, the study by Barendrecht et al [3] was a post hoc analysis. The major limitation of this study was the evaluation of a single AB [3]. To the best of our knowledge, we have performed the first meta-analysis aimed at investigating the effects of ABs on BOOI and other urodynamic measures of BPO in patients with LUTS/BPE. The main finding from the present review is that ABs act as a class by improving urodynamic measurement of BPO in this subset of patients. Indeed, the overall pooled analysis for the studies included showed a net and clinically significant reduction in BOOI after therapy with ABs with respect to baseline values. Although most studies included were not RCTs, our sensitivity analysis confirmed a significant reduction in BOOI in both non-RCTs and RCTs. Moreover, to test for the existence of a potential placebo effect, we performed a subgroup analysis of RCTs with a placebo arm; this confirmed a significant improvement in BOOI in the active treatment arm compared to the placebo arm. The subgroup analysis for single ABs showed a reduction in BOOI for all drugs evaluated. Although no direct comparisons have ever been performed among different ABs, the highest levels of BOOI improvement were reported in the studies on silodosin, which differs from other ABs in its high pharmacologic selectivity for the α1a receptor subtype. However, if and how urodynamic efficacy depends on pharmacologic selectivity is still to be verified. Interestingly, results from the meta-regression analysis demonstrated that AB urodynamic outcomes in terms of BPO depend on the percentage of men with obstruction at baseline. Indeed, the mean BOOI reduction was significantly correlated with the number of patients with obstruction. As a consequence, patients with obstruction should be regarded as the subpopulation that could benefit the most from AB therapy. Notably, PFS is not routinely performed in clinical practice or in most studies reporting the efficacy of ABs in men with LUTS/BPE, and patients are “suspected” of having BPO if free Qmax is <15 ml/s. However, a threshold free Qmax value of 15 ml/s has a positive predictive value of only 67% for BPO, which means that approximately one third of men treated with ABs do not really have obstruction [26].
According to the EAU guidelines, free uroflowmetry may be performed in the initial assessment of male LUTS and should be performed before any treatment (level of evidence [LE] 2b; grade of recommendation [GR] B) [2]. Guidelines recommend PFS only in individual patients for specific indications before surgery or when evaluation of the pathophysiology underlying LUTS is warranted (LE 3; GR B) [2]. As a consequence, for the population of patients taking ABs in everyday clinical practice and in many clinical studies, selection according to free uroflowmetry is not homogeneous in terms of BPO, and the results obtained may provide a poorly realistic picture of AB urodynamic outcomes in terms of BPO.
BOOI is computed using two urodynamic parameters, PdetQmax and Qmax, evaluated during PFS (BOOI = PdetQmax – 2Qmax) [6]. We investigated separately the urodynamic effects induced by ABs on both parameters. Interestingly, the overall pooled data and data for subgroup analyses for single ABs consistently showed a reduction in PdetQmax and an increase in Qmax. However, while the overall change in PdetQmax was robust and clinically relevant (mean change –11.39 cm H2O), the improvement in Qmax could be perceived as clinically marginal (mean increase 2.57 ml/s). Moreover, after pooling data from RCTs that included placebo arms, we found no significant improvement in Qmax in the experimental arm compared with the placebo arm. Taken together, these results support the hypothesis that ABs reduce BOOI mainly by reducing detrusor pressure during voiding, with minimal or no effect on Qmax. This finding has relevant clinical implications. According to the 2015 EAU guidelines on non-neurogenic male LUTS, recommended tests at follow-up visits for patients receiving ABs are the International Prostate Symptom Score (IPSS), free Qmax, and PVR [2]. In addition, most studies evaluating AB therapy for LUTS/BPE consider IPSS, free Qmax, and PVR as the only efficacy measures. Interestingly, in most ABs trials showing strong improvements in symptoms, statistical improvements in free Qmax are considered clinically irrelevant, especially if compared to an increase in free Qmax after surgical treatment for BPO, raising more than a doubt regarding the effect of ABs on obstruction [2], [25], and [27]. However, according to the present meta-analysis, in patients taking ABs a minimal increase in free Qmax can also correspond to a relevant decrease in PdetQmax and BOOI, and indicate clinically meaningful improvements in BPO.
From a pathophysiologic viewpoint, the increase in PdetQmax reported for men with BPO can be regarded as an adaptation to increased outflow resistance. It seems plausible that an inverse adaptation may also take place and that a reduction in BPO translates to lower detrusor pressure at voiding rather than to increased Qmax.
The limits of this meta-analysis reflect the main drawbacks related to the literature on this topic. The few available studies were often outdated and enrolled a small number of patients, and only three RCTs were of good methodological quality. Furthermore, the main endpoint of the analysis was, in most cases, changes in BOOI versus baseline. This test-retest measure does not take into account the placebo effect and might overestimate final outcomes. However, in the three placebo-controlled studies available in the literature, placebo was ineffective in improving BOOI, leading us to hypothesize that such a measure is not affected by any placebo effect. Other limits are the different populations enrolled, the different drugs, and the varying duration of treatment in the different studies included. Finally, very few studies allowed extraction of data on changes in patient classification from one obstruction class to another. Nevertheless, all studies analyzed consistently showed strong improvement in BPO parameters induced by ABs. As the same effect is maintained under variable conditions, this might reinforce our conclusion that ABs induce reliable, relevant improvements in BPO urodynamic parameters.
ABs efficiently improve BPO in men with LUTS/BPE. This effect is higher in patients presenting with urodynamic obstruction at baseline. The free Qmax variation underestimates the real effect of ABs on BPO, as small improvements in Qmax may correspond to relevant improvements in BOOI.
Author contributions: Ferdinando Fusco had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fusco, Creta.
Acquisition of data: Palmieri.
Analysis and interpretation of data: Ficarra, Fusco.
Drafting of the manuscript: Creta, Giannarini.
Critical revision of the manuscript for important intellectual content: Mirone, Longo.
Statistical analysis: Fusco, Verze.
Obtaining funding: None.
Administrative, technical, or material support: Longo, Palmieri.
Supervision: Mirone, Novara.
Other: None.
Financial disclosures: Ferdinando Fusco certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Fabrizio D’Ascenzo, Division of Cardiology, University of Turin, Italy, for his valuable contribution to the statistical analysis.